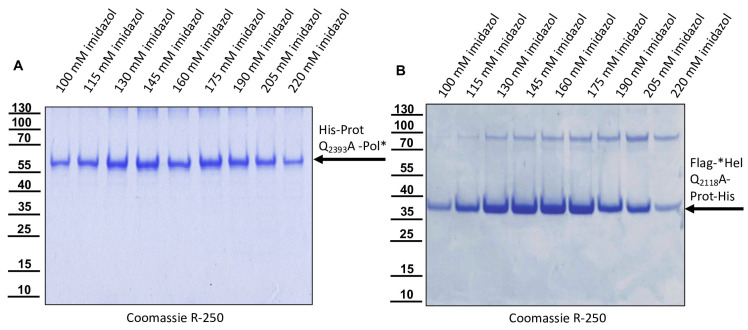
Figure 12.
No cleavage products could be purified from the cleavage site mutants ProtQ2393A-Pol*-His and Flag-*HelQ2118A-Prot-His. (A) The insoluble fraction of a lysate from induced cultures of E. coli transformed with the plasmid pet11a-ProtQ2393A-Pol*-His was solubilized in 8 M urea buffer and subjected to IMAC. The individual imidazole elution fractions were resolved via SDS-PAGE and stained. Note the single protein species with an apparent molecular mass of 60 kDa (ProtQ2393A-Pol*-His) that specifically eluted with increasing imidazole concentrations and the absence of a 28 kDa band (Pol*-His). (B) The insoluble fraction of a lysate from induced cultures of E. coli transformed with the plasmid pet11a-Flag-*HelQ2118A-Prot-His was solubilized in 8 M urea buffer and subjected to IMAC. The individual imidazole elution fractions were resolved via SDS-PAGE and stained. Note the single protein species with an apparent molecular mass of 38 kDa (Flag-*HelQ2118A-Prot-His) that specifically eluted with increasing imidazole concentrations and the absence of a 35 kDa band (Prot-His).