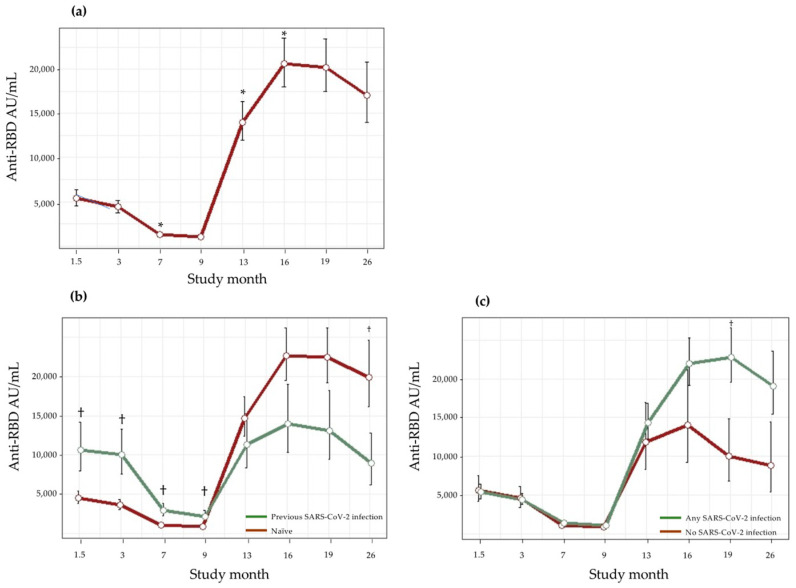
Figure 2.
Evolution of anti-RBD antibodies during follow-up. (a) Anti-RBD antibodies in all study participants; (b) Anti-RBD antibodies in individuals infected and uninfected (naïve) before the first dose of BNT162b2 or prior to the 1.5-month time point; (c) Anti-RBD antibodies in subjects acquiring SARS-CoV-2 infection anytime during follow-up and in naïve subjects who remained uninfected throughout the entire study (see text for additional details). * Significant difference (p < 0.05) compared with the previous time point; † Significant difference (p < 0.05) among groups at indicated time points.