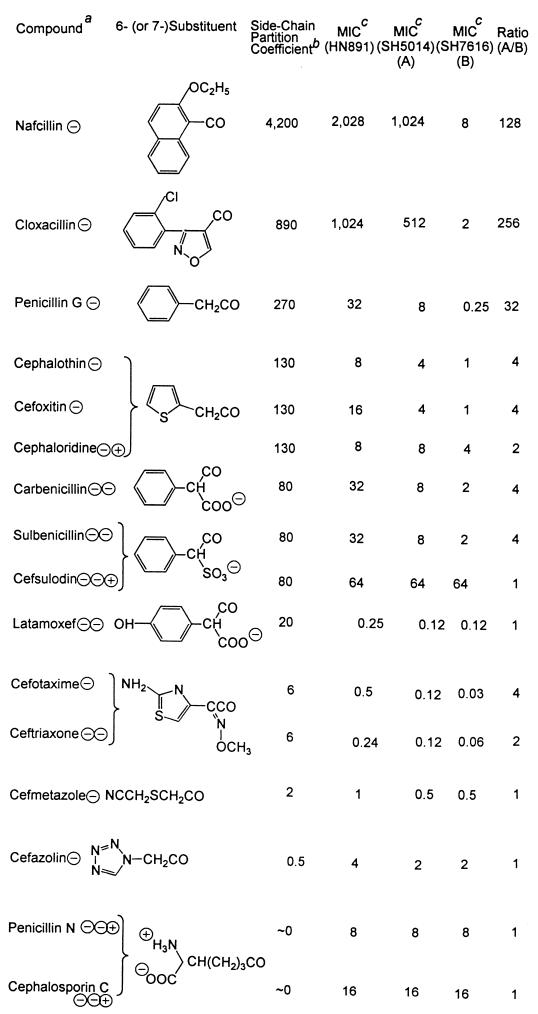
TABLE 2.
Lipophilicities of the side chains and the MICs of penicillins and cephalosporins
The electrical charges present on each compound at a neutral pH are shown after the name of the compound.
b This column shows the calculated octanol-water partition coefficients of the 6-substituents (in penicillins) and the 7-substituents (in cephalosporins). The terminal carbonyl group was excluded from calculations of partition coefficients. When charged groups were present close to the carbonyl group, as in carbenicillin, sulbenicillin, and cefsulodin, partition coefficients were calculated only on the part of the substituent that was farther away from the charged group; that is, calculation included only the phenyl group. Calculation was performed as follows. First, the logarithm of the partition coefficient (logP) was calculated by the fragment approach (31), based on the measured octanol-water partition coefficient of various compounds (10). For example, the calculation of the logP value of the benzyl group in penicillin G is 2.13 (logP of benzene) − 0.225 (fragmental constant for H) + 0.528 (fragmental constant for −CH2−) = 2.43. The logP value of the side chain for cefotaxime and ceftriaxone was calculated by starting from the measured logP value for O-methylbenzaldoxime (2.53) and comparing the logP value of benzene (2.13) to that of 2-aminothiazole (0.38). Partition coefficients were then calculated from logP values.
MICs are in milligrams per liter.