Abstract
Background
Evidence on the durability of the protection of a fourth dose of a monovalent or bivalent messenger ribonucleic acid (mRNA) vaccine against coronavirus disease 2019 (COVID-19) among older people during the predominant Omicron period is needed.
Methods
We performed a population-based cohort study in Norway covering the time from 1 July 2022 to 15 January 2023, including individuals ≥75 years of age who had received at least a third dose. Using Cox proportional hazard models on severe COVID-19-associated outcome measures and all-cause mortality, we estimated the vaccine effectiveness of mono- and bivalent vaccines, comparing fourth- to third-dose recipients (>24 weeks ago). Vaccine status was included as a time-varying covariate and models were adjusted for potential confounders.
Results
We included 408 073 individuals. A fourth dose with either monovalent or bivalent mRNA vaccine showed increased protection against COVID-19-associated mortality relative to a third dose in individuals ≥75 years of age. We estimated a protective effect for the bivalent BA.1 vaccine [adjusted hazard ratio (aHR) 0.08, 95% CI 0.02–0.32] relative to the bivalent BA.4–5 (aHR 0.27, 95% CI 0.14–0.56) and a monovalent dose (aHR 0.34, 95% CI 0.26–0.45) 2–9 weeks after vaccination compared with recipients with a third dose >24 weeks ago. The increased protective effect waned with no added protection for the monovalent vaccine after 33 weeks compared with a third dose.
Conclusions
Our results indicate an increased protective effect of a fourth dose against severe outcomes compared with a third dose, with decreasing effect with time since the last dose.
Keywords: Omicron, SARS-CoV-2, vaccine effectiveness, booster dose, bivalent vaccine
Key Messages.
A fourth dose with a monovalent or bivalent vaccine (BA.1 or BA.4–5) provided additional protection against severe coronavirus disease 2019 (COVID-19)-related outcomes and all-cause mortality compared with only a third dose (>24 weeks ago) among individuals ≥75 years old in Norway.
Our results indicate a waning protective effect of the fourth dose with a monovalent vaccine compared with the third dose, starting 10–17 weeks after vaccination.
After 33 weeks, there was no additional protective effect of a fourth dose with a monovalent vaccine on severe COVID-19-related outcomes compared with a third dose (>24 weeks ago).
The waning of a fourth dose seems important to consider when defining future COVID-19 booster vaccination strategies.
Introduction
Since December 2021, Omicron (B.1.1.529) has become the dominant severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant globally and a rapid increase in new coronavirus disease 2019 (COVID-19) cases was observed.1
Previous studies identified that the Omicron variants BA.4 and BA.5 show a substantial escape from vaccine or infection-induced neutralizing antibodies.2,3 Thus, the initial monovalent messenger ribonucleic acid (mRNA) booster vaccines based on the original virus (Wuhan) may not provide a long-lasting protection against the new emerging variants.4–6 However, the immune evasion seems to be less pronounced in individuals with hybrid immunity resulting from prior infection and vaccination.4,7 To combat the weakened effectiveness of the monovalent BNT162b2 (Comirnaty, Pfizer-BioNTech) and mRNA-1273 (Spikevax, Moderna) COVID-19 vaccines, the bivalent mRNA booster vaccines, covering the original Wuhan virus and BA.4–5 or BA.1, were recently made available in most countries and implemented in the booster vaccination programmes, including for most vulnerable groups such as individuals aged ≥75 years as well as residents of long-term care facilities (LTCFs). The bivalent vaccines are expected to offer a broader immune response able to neutralize Omicron lineages.8 Previous vaccine effectiveness (VE) studies of fourth doses mainly focused on the monovalent mRNA COVID-19 vaccines and periods prior to the emergence of the subvariants BA.4 and BA.5. Early results indicate that vaccination with the bivalent mRNA boosters as a fourth vaccine dose (second booster) increases protection against COVID-19-associated hospitalization and mortality compared with only receiving three doses (first booster).9,10 In Norway, the fourth dose of COVID-19 vaccine became available in April 2022 but was not yet recommended for anyone. It was not until 1 July 2022 that a fourth dose was recommended for the most vulnerable groups, including those aged ≥75 years and residents of LTCFs.11,12 Since September 2022, the adapted bivalent vaccines Comirnaty and Spikevax have become available in the COVID-19 vaccination programme in Norway.11
Real-world evidence on the durability of the protection and the effectiveness of the bivalent booster vaccines on severe COVID-19 outcome is needed. Therefore, our study aimed to compare the added protective effect of the fourth dose and the bivalent vaccines against COVID-19-associated mortality and all-cause mortality, COVID-19-associated hospitalization, as well as intensive care unit (ICU) admission among individuals ≥75 years of age in Norway.
Methods
Data collection and study population
We conducted a population-based cohort study and linked data from the National Emergency Preparedness Register (Beredt C19), which contains individual-level data from various registries in Norway. More details about the different data sources and Beredt C19 are provided in the Supplementary Table S2 (available as Supplementary data at IJE online). Relevant data was extracted on 13 February covering the study period from 1 July 2022 until 15 January 2023; 1 July 2022 was chosen because this is the date on which when the fourth dose was recommended for the most vulnerable groups in Norway.12 We included all individuals aged ≥75 years with a national identity number in the Norwegian national registry who had received at least three vaccine doses by 1 July 2022, which comprised the base population. Individuals were excluded based on pre-defined criteria displayed in Figure 1 and Supplementary Text S1 (available as Supplementary data at IJE online).
Figure 1.
Flow chart of included individuals who contributed time at risk in our analyses
Definition of outcome measures
The primary outcome measures in our study were (i) COVID-19-associated mortality and (ii) all-cause mortality, including individuals who died of other causes. Secondary outcome measures were (iii) COVID-19-associated hospitalization and (iv) COVID-19-associated ICU admission. More detailed information on definitions and adjustment variables is provided in Supplementary Text S1 and Table S1 (available as Supplementary data at IJE online).
Statistical analyses
Descriptive analyses were performed to present the socio-demographic characteristics of the overall study population as well as stratified by individuals who received a third dose and/or a fourth dose over the study period. In addition, we calculated the number of severe COVID-19-associated outcome measures and all-cause mortality during the study period. To provide more detailed information about the time at risk, we reported the number of person-years provided by individuals at risk until censoring or the occurrence of each event, stratified by the vaccine dose that individuals received.
Cox proportional hazard models were performed using the coxph function from the survival R-package13 to estimate the adjusted hazard ratios (aHRs) on the primary and secondary outcomes measures. We estimated aHRs for individuals who received a fourth dose compared with those who had received the third dose >24 weeks ago. Vaccination status, a combination of time under risk since the last dose and vaccine type (for the fourth dose), was the main predictor and included in the model as a time-varying variable (more details on this time-varying variable are provided in Supplementary Table S3, available as Supplementary data at IJE online).14 All models were stratified using pre-defined strata of age groups, sex, region of residence, risk group and whether an individual was a resident in a LTCF by applying strata (function) of the R survival package.13 This allows the covariate combination to have non-proportional differences, i.e. groups are not assumed to have the same baseline hazard over time. The aHR was reported for all levels of the vaccine status factor. As subgroup analysis, we performed an additional Cox proportional hazard model that we stratified by LTCF residents to assess potential differences of lower vaccine-induced protection against the outcome measures among older people living at home and those living in LTCFs. The results are presented as aHRs with 95% confidence intervals (CIs). All analyses were performed using R Version 4.0.2.
Results
Study population
We included a total of 408 073 individuals, of whom 55.7 % (227 290) were female. A total of 23 209 individuals had already received a fourth dose by 1 July 2022. The median time to receive the fourth dose during the study period was 32 days (interquartile range 17–42 days). Detailed socio-demographic characteristics of the study population at baseline and by the vaccine dose that individuals received during the study period are shown in Table 1. Overall, a fourth dose was received by 319 582, of whom 81.1% (259 179) received a monovalent and 18.9% (60 403) a bivalent vaccine. Among bivalent recipients, 57.8% (34 881) and 42.3% (25 522) received a BA.1 and BA.4–5 dose, respectively. The proportion of individuals in the oldest age groups (>85 years) was slightly higher among monovalent recipients (24.8%; 64 007) compared with those who received a bivalent vaccine (BA.1: 18.8%; 6552; BA.4–5: 21.5%; 5494). The groups were comparable regarding high- and medium-risk groups, displayed in Table 1.
Table 1.
Socio-demographic characteristics of the study population and stratified for individuals who received a third dose and/or a fourth dose over the study period
| Study population | Third-dose recipients a | Fourth-dose recipients |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
Monovalent vaccine |
BA.1 bivalent vaccine |
BA.4–5 bivalent vaccine |
||||||
| n | % | n | % | n | % | n | % | n | % | |
| Sex | ||||||||||
| Female | 227 290 | 55.70 | 214 120 | 55.60 | 142 665 | 55.04 | 18 621 | 53.38 | 13 781 | 54.00 |
| Male | 180 783 | 44.30 | 170 744 | 44.40 | 116 514 | 44.96 | 16 260 | 46.62 | 11 741 | 46.00 |
| Age (years) | ||||||||||
| 75–79 | 194 594 | 47.70 | 191 434 | 49.70 | 122 884 | 47.41 | 19 733 | 56.57 | 13 589 | 53.24 |
| 80–84 | 111 271 | 27.30 | 100 959 | 26.20 | 72 288 | 27.89 | 8596 | 24.64 | 6439 | 25.23 |
| 85–89 | 63 264 | 15.50 | 57 243 | 14.90 | 39 867 | 15.38 | 4355 | 12.49 | 3524 | 13.81 |
| 90+ | 38 944 | 9.50 | 35 228 | 9.20 | 24 140 | 9.31 | 2197 | 6.30 | 1970 | 7.72 |
| Region | ||||||||||
| Innlandet | 36 022 | 8.80 | 33 868 | 8.80 | 22 451 | 8.66 | 3808 | 10.92 | 2072 | 8.12 |
| Trøndelag | 36 702 | 9.00 | 34 878 | 9 | 23 618 | 9.00 | 3038 | 9.00 | 1807 | 7.00 |
| Nord-Norge | 39 775 | 9.70 | 38 615 | 10.00 | 20 636 | 7.96 | 3683 | 10.56 | 3264 | 12.79 |
| Oslo & Viken | 133 582 | 32.70 | 123 735 | 32.20 | 87 962 | 33.94 | 10 611 | 30.42 | 9809 | 38.43 |
| Agder & Sørøstlandet | 60 375 | 14.80 | 56 980 | 14.80 | 38 368 | 14.80 | 5074 | 14.55 | 4052 | 15.88 |
| Vestlandet | 101 617 | 24.90 | 96 788 | 25.10 | 66 144 | 25.52 | 8667 | 24.85 | 4518 | 17.70 |
| Risk group | ||||||||||
| None | 189 581 | 46.50 | 179 726 | 46.70 | 120 725 | 46.58 | 16 549 | 47.44 | 11 973 | 46.91 |
| Medium | 187 608 | 46.10 | 176 342 | 45.80 | 118 653 | 45.78 | 15 865 | 45.48 | 11 706 | 45.87 |
| High | 30 884 | 7.60 | 28 796 | 7.50 | 19 801 | 7.64 | 2467 | 7.07 | 1843 | 7.22 |
| LTCF resident | ||||||||||
| No | 388 227 | 95.10 | 367 392 | 95.50 | 244 997 | 94.53 | 34 472 | 98.83 | 25 086 | 98.29 |
| Yes | 19 846 | 4.90 | 17 472 | 4.50 | 14 182 | 5.47 | 409 | 1.17 | 436 | 1.71 |
LTCF, long-term care facility. n, number.
Individuals who received their third dose >24 weeks ago.
Amongst 408 073 individuals, 732 (0.2%) deaths were associated with COVID-19 and 13 430 (3.3%) deaths were not associated with COVID-19. A total of 2613 (0.6%) individuals were hospitalized with COVID-19 as the main reason for admission and 110 (0.03%) individuals were admitted to the ICU. More detailed information about the time at risk until each event occurred stratified by vaccine dose is displayed in Table 2 and Supplementary Table S4 (available as Supplementary data at IJE online).
Table 2.
Socio-demographic characteristics by primary outcome measure of COVID-19-associated mortality and all-cause mortality, with the time under risk in person-years for each event stratified by the vaccine dose
|
Third-dose recipients
a
|
Fourth-dose recipients |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monovalent vaccine |
BA.1 bivalent vaccine |
BA.4–5 bivalent vaccine |
||||||||||||||||||||||
|
Person-time
b
|
COVID-19-associated mortality |
All-cause mortality |
Person-time
b
|
COVID-19-associated mortality |
All-cause mortality |
Person-time
b
|
COVID-19-associated mortality |
All-cause mortality |
Person-time
b
|
COVID-19-associated mortality |
All-cause mortality |
|||||||||||||
| Years | % | n | % | n | % | Years | % | n | % | n | % | Years | % | n | % | n | % | Years | % | n | % | n | % | |
| Sex | ||||||||||||||||||||||||
| Female | 50 834 | 56.80% | 194 | 47.90% | 3503 | 53.10% | 61 365 | 55.22% | 154 | 49.52% | 3678 | 57.17% | 5314 | 53.44% | 4 | 50.0% | 115 | 48.12% | 2522 | 54.07% | 2 | 25.0% | 82 | 51.25% |
| Male | 38 718 | 43.20% | 211 | 52.10% | 3095 | 46.90% | 49 758 | 44.78% | 157 | 50.48% | 2755 | 42.83% | 4630 | 46.56% | 4 | 50.0% | 124 | 51.88% | 2143 | 45.93% | 6 | 75.0% | 78 | 48.75% |
| Age (years) | ||||||||||||||||||||||||
| 75–79 | 43 998 | 49.10% | 79 | 19.50% | 1510 | 22.90% | 51 701 | 46.53% | 57 | 18.33% | 1057 | 16.43% | 5648 | 56.80% | 2 | 25.0% | 61 | 25.52% | 2502 | 53.64% | 4 | 50.0% | 42 | 26.25% |
| 80–84 | 23 589 | 26.30% | 70 | 17.30% | 1446 | 21.90% | 31 723 | 28.55% | 58 | 18.65% | 1346 | 20.92% | 2444 | 24.57% | 2 | 25.0% | 41 | 17.15% | 1169 | 25.07% | 0 | – | 36 | 22.50% |
| 85–89 | 13 689 | 15.30% | 102 | 25.20% | 1605 | 24.30% | 17 388 | 15.65% | 82 | 26.37% | 1651 | 25.66% | 1230 | 12.37% | 1 | 12.5% | 73 | 30.54% | 636 | 13.63% | 2 | 25.0% | 42 | 26.25% |
| 90+ | 8275 | 9.20% | 154 | 38.00% | 2037 | 30.90% | 10 311 | 9.28% | 114 | 36.66% | 2379 | 36.98% | 623 | 6.26% | 3 | 37.5% | 64 | 27.00% | 358 | 8.00% | 2 | 25.0% | 40 | 25.00% |
| Region | ||||||||||||||||||||||||
| Innlandet | 8305 | 9.30% | 33 | 8.10% | 592 | 9.00% | 9157 | 8.00% | 31 | 9.97% | 570 | 8.86% | 1145 | 11.51% | 3 | 37.5% | 29 | 12.13% | 409 | 8.76% | 0 | – | 18 | 11.25% |
| Trøndelag | 8205 | 9.20% | 27 | 6.70% | 604 | 9.20% | 10 046 | 9.04% | 27 | 8.68% | 596 | 9.26% | 847 | 8.52% | 1 | 12.5% | 23 | 9.62% | 281 | 6.01% | 1 | 12.5% | 10 | 6.25% |
| Nord-Norge | 10 978 | 12.30% | 40 | 9.90% | 739 | 11.20% | 8403 | 7.56% | 22 | 7.07% | 514 | 7.99% | 1000 | 10.06% | 0 | 0.0% | 16 | 6.69% | 565 | 12.11% | 0 | – | 16 | 10.00% |
| Oslo & Viken | 27 028 | 30.20% | 132 | 32.60% | 1948 | 29.50% | 38 520 | 34.66% | 109 | 35.05% | 2155 | 33.50% | 3004 | 30.21% | 2 | 25.0% | 68 | 28.45% | 1924 | 41.25% | 3 | 37.5% | 54 | 33.75% |
| Agder & Sørøstlandet | 13 006 | 14.50% | 46 | 11.40% | 975 | 14.80% | 16 673 | 15.00% | 39 | 12.54% | 975 | 15.16% | 1473 | 14.81% | 0 | – | 27 | 11.30% | 739 | 15.85% | 3 | 37.5% | 39 | 24.38% |
| Vestlandet | 22 031 | 24.60% | 127 | 31.40% | 1740 | 26.40% | 28 324 | 25.49% | 83 | 26.69% | 1623 | 25.23% | 2475 | 24.89% | 2 | 25.0% | 76 | 31.80% | 747 | 16.01% | 1 | 12.5% | 23 | 14.38% |
| Risk group | ||||||||||||||||||||||||
| None | 42 089 | 47.00% | 120 | 29.60% | 1929 | 29.20% | 51 804 | 46.62% | 113 | 36.00% | 2010 | 31.00% | 4741 | 48.00% | 3 | 37.5% | 76 | 31.80% | 2211 | 47.40% | 2 | 25.0% | 46 | 28.75% |
| Medium | 41 036 | 45.80% | 220 | 54.30% | 3622 | 54.90% | 50 849 | 45.76% | 165 | 53.05% | 3555 | 55.26% | 4505 | 45.31% | 4 | 50.0% | 126 | 52.72% | 2120 | 45.45% | 5 | 62.5% | 91 | 56.88% |
| High | 6427 | 7.20% | 65 | 16.00% | 1047 | 15.90% | 8469 | 7.62% | 33 | 10.61% | 868 | 13.49% | 698 | 7.02% | 1 | 12.5% | 37 | 15.00% | 334 | 7.00% | 1 | 12.5% | 23 | 14.00% |
| LTCF resident | ||||||||||||||||||||||||
| No | 86 319 | 96.40% | 277 | 68.40% | 5096 | 77.20% | 10 5061 | 94.55% | 163 | 52.41% | 3999 | 62.16% | 9829 | 98.85% | 8 | 100.0% | 213 | 89.12% | 4592 | 98.44% | 8 | 100.0% | 137 | 85.63% |
| Yes | 3233 | 3.60% | 128 | 31.60% | 1502 | 22.80% | 6061 | 5.45% | 148 | 47.59% | 2434 | 37.84% | 115 | 1.16% | 0 | – | 26 | 10.88% | 73 | 1.56% | 0 | – | 23 | 14.38% |
LTCF, long-term care facility; COVID-19, coronavirus disease 2019; n, number.
Individuals who received their third dose >24 weeks ago.
The time under risk for third- and fourth-dose recipients until censoring or occurrence of the respective event.
The overall numbers of COVID-19-associated deaths, all-cause mortality, COVID-19-associated hospitalization and ICU admission during the study period in Norway is displayed in the epidemic curve in Supplementary Figure S1 (available as Supplementary data at IJE online) and the dominating SARS-CoV-2 variants in Norway in Supplementary Figure S2 (available as Supplementary data at IJE online).
Estimates of protection for COVID-19-associated mortality and all-cause mortality
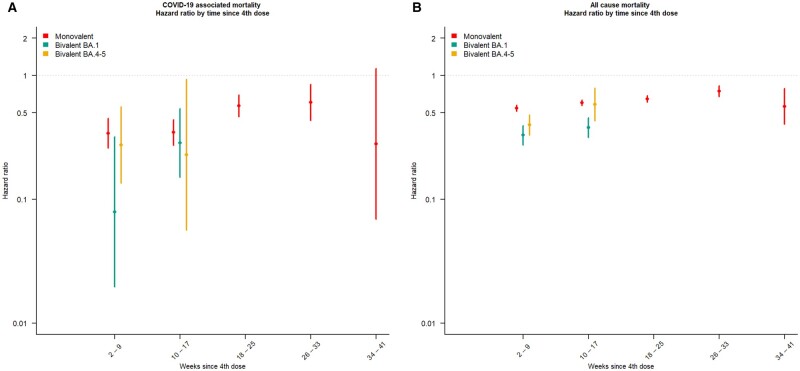
The results of our Cox-regression analyses indicating an additional protective effect against COVID-19-associated mortality of a fourth dose for mono- and bivalent vaccines compared with having received a third dose >24 weeks ago, as all aHRs were <1. A fourth dose of both monovalent and bivalent vaccines show a high protection against COVID-19-associated mortality 2–9 weeks after vaccination. Although not statistically significant, a numerically higher protection was conferred by the BA.1 (aHR 0.08, 95% CI 0.02–0.32) relative to the BA.4–5 (aHR 0.27, 95% CI 0.14–0.56) and a monovalent dose (aHR 0.34, 95% CI 0.26–0.45), as displayed in Figure 2A. A protective effect of a fourth dose relative to a third dose was also identified for the outcome of all-cause mortality (Figure 2B). Receiving a bivalent fourth dose indicate a little higher protective effect (BA.1: aHR 0.33, 95% CI 0.28–0.40; BA.4–5: aHR 0.40, 95% CI 0.33–0.48) during weeks 2–9 after vaccination compared with a monovalent vaccine (aHR 0.55; 95% CI 0.52–0.58).
Figure 2.
The adjusted hazard ratio (aHR) estimates for (A) COVID-19-associated mortality and (B) all-cause mortality by vaccination with an mRNA booster among all individuals living in Norway with a valid national identity number and aged ≥75 years. Fourth dose, >7 days after a fourth COVID-19 vaccine dose was given with either a monovalent or bivalent (BA.1 or BA.4–5) vaccine. ≥24 weeks since the third dose was used as reference level. The Cox proportional hazard model is stratified for age, sex, risk group, resident in long-term care facility and county of residence. Due to the recent introduction of the BA.1 and BA.4–5 vaccines, estimates could only be calculated for the monovalent vaccines for all vaccination statuses
The protective effect waned over time for both primary outcome measures. After 33 weeks we did not identify an additional protective effect of a fourth dose with a monovalent vaccine on COVID-19-associated mortality (aHR 0.28, 95% CI 0.07–1.14) compared with a third dose >24 weeks ago (Figure 2A and B). The bivalent vaccines were implemented recently and therefore we could not estimate the protective effect after >17 weeks.
The results of the subgroup analysis for LTCF residents did not show any differences for the protective effect of COVID-19-associated mortality and all-cause mortality among individuals who received a fourth dose with a monovalent vaccine. The results are shown in Supplementary Table S5 (available as Supplementary data at IJE online).
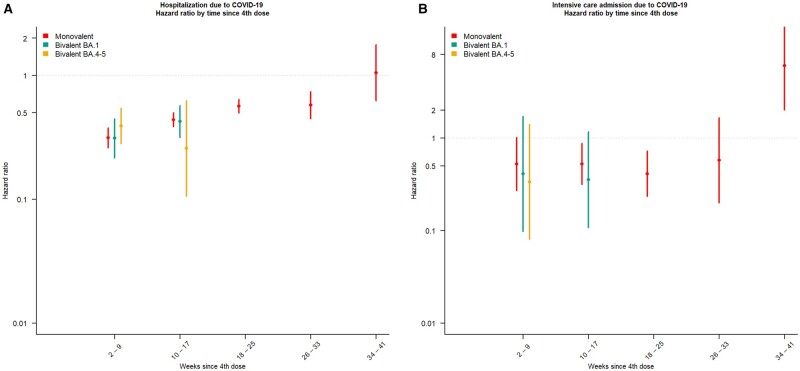
Estimates of protection for COVID-19-associated hospitalization and ICU admission
Estimates for the secondary outcome measures of COVID-19-associated hospitalization and ICU admission were comparable to the primary outcome measures, elucidating a protective effect of a fourth dose compared with a third dose. We identified a waning of the protective effect for hospitalization after 17 weeks for the monovalent vaccines, with an aHR of 0.31 (95% CI 0.26–0.38) after 2–9 weeks to an aHR of 0.58 (95% CI 0.45–0.73) after 26–33 weeks (Figure 3A). The waning of the protective effect was less pronounced for ICU admission with greater uncertainty of the estimates (Figure 3B). Our results indicate that the protective effect seems to be slightly more pronounced for COVID-19-associated hospitalization compared with COVID-19-associated ICU admission during 2–9 weeks after vaccination. We did not identify differences for the protective effect against ICU admission when comparing bivalent BA.1 with BA.4–5 and monovalent doses (Figure 3A and B).
Figure 3.
The adjusted hazard ratio (aHR) estimates for COVID-19-associated (A) hospitalization and (B) intensive care unit admission by vaccination with an mRNA booster among all individuals living in Norway with a valid national identity number and aged ≥75 years. Fourth dose, >7 days after a fourth COVID-19 vaccine dose was given with either a monovalent or bivalent (BA.1 or BA.4–5) vaccine. The results of the Cox proportional hazard model are stratified for age, sex, risk group, resident in a long-term care facility and county of residence. Due to the recent introduction of the BA.1 and BA.4–5 vaccines, estimates could only be calculated for the monovalent vaccines
Discussion
Our population-based cohort study among individuals aged ≥75 years in Norway shows that a fourth dose with a monovalent or bivalent vaccine provided additional protection against severe COVID-19-related outcomes and all-cause mortality than a third dose >24 weeks ago during an Omicron dominating period (Supplementary Figure S2, available as Supplementary data at IJE online).
The current recommendations for the bivalent COVID-19 vaccines are mainly based on evidence from immunogenicity studies indicating a greater response against current circulating Omicron lineages.15,16 But current evidence on the real-world effectiveness of the fourth COVID-19 dose and bivalent vaccines in older people is sparse. Our population-based study provides complementary evidence to existing studies because of the larger study population and longer follow-up.9,17 In addition, we were able to compare the effect of the different vaccines, BA.1, BA.4–5 and the monovalent vaccine used for the fourth dose over time.
Protective effect for COVID-19-associated outcome measures and all-cause mortality
In our study, we identified high protection against severe COVID-19-associated outcome measures and all-cause mortality for both monovalent and bivalent vaccines (BA.1 and BA.4–5) ≤17 weeks after vaccination, in a period when various Omicron lineages circulated predominantly in Norway. These findings are consistent with previous studies, although different methods, definitions and follow-up times were used, which indicate similar results regarding the protective durability among older people and most vulnerable groups.17–20 Comparable levels of protection were identified in a cohort study from Israel covering a shorter follow-up period, which estimated a protective effect for COVID-19-associated mortality (aHR 0.32, 95% CI 0.18–0.58) and hospitalization (aHR 0.28, 95% CI 0.19–0.40) in individuals aged ≥65 years for ≤120 days by comparing individuals who received a bivalent vaccine with those who were unvaccinated.9 A recent study from Sweden compared the VE of a third and fourth dose against all-cause mortality in individuals aged >80 years and reported a greater protective effect in those living in a LTCF (61%, 95% CI 58–64%) compared with those living at home (50%, 95% CI 46–54%).17 Whereby stratifying our analysis according to residential status did not appreciably impact our results for a monovalent vaccine on COVID-19-associated mortality and all-cause mortality. Since the group of LTCF residents were prioritized in the vaccination programme and therefore were vaccinated before the bivalent vaccines were introduced, the number of LTCF residents with bivalent vaccines was very low and therefore the protective effect could not be estimated.
The protective effect for all-cause mortality identified in the Swedish study17 was also found in our analyses. Even though our models were adjusted for demographic data and risk factors for severe COVID-19-associated outcomes, which decreases the risk of bias, we cannot rule out that the protective effect estimates might be overestimated due to the healthy vaccinee bias.21,22 As all-cause mortality is a relatively unspecific outcome measure, our results can also be biased due to factors that we insufficiently corrected for in our models.
During the winter season, viral infections such as influenza and respiratory syncytial virus are more prevalent, with an increased risk of mortality, particularly among older people and individuals with underlying health conditions. Thus, the results might indicate that vaccination with the updated vaccines before the winter could have provided additional protection against all-cause mortality during the winter season.
Waning of the protective effect against COVID-19-associated severe outcomes
We could not estimate the protective effect of the bivalent vaccine for >17 weeks after vaccination due to the recent introduction of these vaccines. Additionally, the estimated effect for the bivalent vaccines can be biased for the time 10–17 weeks after vaccination due to a different time under risk. Some bivalent recipients in our study had a shorter time at risk and therefore less time for the waning effect to become visible. However, our results indicate a waning protective effect of the fourth dose with a monovalent vaccine compared with the third dose for all vaccines, starting 10–17 weeks after vaccination. A substantial waning immunity after this period was also found in previous studies.17,23 The waning of a fourth dose among older people seems therefore to be important to consider when defining future booster vaccination strategies.24,25
Differences between mono- and bivalent COVID-19 vaccines as fourth dose
Up to now, only limited data have been available to identify differences in VE between bivalent and monovalent vaccines as a fourth dose. Recent findings from North Carolina, USA identified a VE of 61.5% (95% CI 47.1–71.9%) against hospitalization or deaths among individuals aged ≥65 years who received a bivalent dose; the protective effect was 41.2% higher compared with a monovalent dose (20.3%, 95% CI −6.0–40.1%).18 In our study, we did not identify a clear indication that the bivalent vaccines were superior to a monovalent fourth dose against COVID-19-related outcome measures. However, the protective effect with a bivalent COVID-19 vaccine seemed to be generally slightly higher against all-cause mortality compared with a monovalent vaccine. Nevertheless, this outcome measure is potentially more likely to be biased due to other factors that have not been considered in our models.
Even though we did not identify huge differences between baseline characteristics, such as risk group, sex and age, between third- and fourth-dose recipients, the most vulnerable groups had been prioritized for the fourth-dose roll-out and were vaccinated with a monovalent vaccine due to the availability (see Supplementary Figure S3, available as Supplementary data at IJE online), which could also have raised their risk for severe outcome and led to bias of the estimates when comparing mono- and bivalent vaccines.
Some of the latest study findings might indicate that the bivalent vaccines potentially improve and broaden the protection during the Omicron period.9,18 However, more real-world evidence with longer and larger-sized follow-up data is needed. Therefore, continued monitoring will be important to fully understand the durability of protection of the mono- and bivalent vaccines with the emergence of Omicron sub-lineages and new COVID-19 variants over time.
Limitations
Our study has several limitations. First, the limits of a register-based cohort study should be considered when interpreting the results, as the data were not collected for the purpose of our study only. Second, we should consider that in some cases the outcome of COVID-19-associated mortality may have not directly been caused by SARS-CoV-2. To provide a broader picture of our results, we also included a model on all-cause mortality in our analyses. Third, we could not consider prior infections during the Omicron period because mandatory public testing was stopped mid-January 2022, thus data on infections were no longer reliable after this. Therefore, we could not differentiate between the protective effect of hybrid immunity compared with vaccine-only immunity, which could have led to biased VE estimates.26,27 However, we excluded individuals who had been previously hospitalized due to COVID-19 to minimize bias. Fourth, even though the models were adjusted for the presence of underlying risk for a severe COVID-19-associated outcome, the severity of the disease and not only the presence could affect the observed differences of the protective effects. In addition, the number of events was relatively small for mortality and ICU admissions, which could have amplified any differences in the protective effect between monovalent and bivalent vaccines. Fifth, our results present estimates on VE during the predominant Omicron period among individuals ≥75 years old and are not generalizable for other age groups and other time periods. Finally, the number of events in specific strata pertaining to COVID-19-associated mortality and ICU admission outcomes were low, warranting careful consideration when interpreting the results. Nonetheless, the overall protective effect of the COVID-19 vaccines for all outcome measures remained robust during the initial 17 weeks after the fourth dose.
Conclusions
Our analyses provide important evidence on the protective effect and durability of a fourth dose of monovalent and bivalent vaccines BA.1 and BA.4–5 during the predominant Omicron period among individuals ≥75 years of age in Norway. Even though the additional protective effect of the fourth dose waned with time since vaccination, both monovalent and bivalent vaccines showed a high additional protective effect against severe COVID-19 outcomes when compared with a third dose. Our results contribute to the increasing evidence suggesting that the level of protection against severe outcomes seems to wane with the length of time since the last dose within individuals aged ≥75 years.
Ethics approval
This project was performed under the ethics approval that was granted by the Regional Committees for Medical and Health Research Ethics (REC) Southeast (reference number 122745). The Norwegian Institute of Public Health has performed a Data Protection Impact Assessment for Beredt C19.
Supplementary Material
Acknowledgements
We would like to acknowledge the work of the Beredt C19 team at the Norwegian Institute of Public Health and the European Programme for Intervention Epidemiology Training, ECDC, without whom this study would not have been possible.
Contributor Information
Melanie Stecher, Department of Infection Control and Vaccines, Norwegian Institute of Public Health, Oslo, Norway; ECDC Fellowship Programme, Field Epidemiology path (EPIET), European Centre for Disease Prevention and Control (ECDC), Stockholm, Sweden.
Anja Bråthen Kristoffersen, Department of Method Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
Kristian Lie, Department of Infection Control and Vaccines, Norwegian Institute of Public Health, Oslo, Norway.
Svein Rune Andersen, Department of Infection Control and Vaccines, Norwegian Institute of Public Health, Oslo, Norway.
Hinta Meijerink, Department of Infection Control and Vaccines, Norwegian Institute of Public Health, Oslo, Norway.
Jostein Starrfelt, Department of Infection Control and Preparedness, Norwegian Institute of Public Health, Oslo, Norway.
Data availability
The data sets analysed during the current study come from the national emergency preparedness registry for COVID-19 (Beredt C19), housed at the Norwegian Institute of Public Health. The preparedness registry comprises data from a variety of central health registries, national clinical registries and other national administrative registries. Legal restrictions prevent the researchers from sharing the data set used in the study. However, external researchers are freely able to request access to linked data from the same registries from outside the structure of Beredt C19, as per the normal procedure for conducting health research on registry data in Norway (https://www.helsedata.no). Further information on the preparedness registry, including access to data from each data source, is available at https://www.fhi.no/en/id/corona/coronavirus/emergency-preparedness-register-for-covid-19/. Code and model results in summary from R are available from the author.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
J.S., M.H. and M.S. developed the concept and design for the study. J.S. and M.S. performed data analyses, A.B.K. and M.S. verified the underlying data and model code. M.S. and J.S. interpreted the data and drafted the manuscript. All authors had the opportunity to request data access. All authors read and gave critical feedback on the manuscript and approved the final version.
Funding
This study was conducted as part of the Norwegian Institute of Public Health mandate. The author M.S. is a fellow of the ECDC Fellowship Programme, supported financially by the European Centre for Disease Prevention and Control (ECDC). The views and opinions expressed herein do not state or reflect those of the ECDC. The ECDC is not responsible for the data and information collation and analysis and cannot be held liable for conclusions or opinions drawn. There was no external funding source for this study.
Conflict of interest
None declared.
References
- 1. World Health Organization. Tracking SARS-CoV-2 Variants. 2023. https://www.who.int/activities/tracking-SARS-CoV-2-variants (28 February 2023, date last accessed).
- 2. Cele S, Jackson L, Khoury DS. et al. ; COMMIT-KZN Team. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022;602:654–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Iketani S, Guo Y. et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022;602:676–81. [DOI] [PubMed] [Google Scholar]
- 4. Hachmann NP, Miller J, Collier AY. et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med 2022;387:86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edara VV, Manning KE, Ellis M. et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. Cell Rep Med 2022;3:100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyke KE, Atmar RL, Islas CD. et al. ; DMID 21-0012 Study Group. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med 2022;3:100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bobrovitz N, Ware H, Ma X. et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis 2023;23:556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chalkias S, Harper C, Vrbicky K. et al. A bivalent Omicron-containing booster vaccine against Covid-19. N Engl J Med 2022;387:1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arbel R, Peretz A, Sergienko R. et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect Dis 2023;23:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson NW, Thiesson EM, Baum U. et al. Comparative effectiveness of bivalent BA.4-5 and BA.1 mRNA booster vaccines among adults aged ≥50 years in Nordic countries: nationwide cohort study. 2023;382:e075286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norwegian Institute of Public Health. Coronavirus Vaccine—Information for the Public. 2023. https://www.fhi.no/en/id/vaccines/coronavirus-immunisation-programme/coronavirus-vaccine/#booster-doses (28 February 2023, date last accessed).
- 12. Norwegian Institute of Public Health. Informasjonsbrev nr 47 om Koronavaksinasjonsprogrammet [Information Letter No. 47 About the Corona Vaccination Programme].2022. https://www.fhi.no/publ/brev/informasjonsbrev-nr-47-om-koronavaksinasjonsprogrammet/ (7 July 2023, date last accessed).
- 13. Therneau TM. A Package for Survival Analysis in R. R Package Version 3.5-5. 2023. https://CRAN.R-project.org/package=survival (7 July 2023, date last accessed).
- 14. European Centre for Disease Prevention and Control. Protocol for a COVID-19 Vaccine Effectiveness Study Using Health Data Registries: Stockholm ECDC. Report No.: 1.0. 2023. https://www.ecdc.europa.eu/en/publications-data/protocol-covid-19-vaccine-effectiveness-study-using-health-data-registries.
- 15. Wang Q, Bowen A, Valdez R. et al. Antibody response to Omicron BA.4-BA.5 bivalent booster. N Engl J Med 2023;388:567–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou J, Kurhade C, Patel S. et al. Neutralization of BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with bivalent vaccine. N Engl J Med 2023;388:854–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordström P, Ballin M, Nordström A.. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: a nationwide, retrospective cohort study in Sweden. Lancet Reg Health Eur 2022;21:100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin DY, Xu Y, Gu Y. et al. Effectiveness of bivalent boosters against severe Omicron infection. N Engl J Med 2023;388:764–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tenforde MW, Weber ZA, Natarajan K. et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-associated emergency department or urgent care encounters and hospitalizations among immunocompetent adults—VISION network, nine states, September-November 2022. MMWR Morb Mortal Wkly Rep 2023;72:579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersson NW, Thiesson EM, Baum U. et al. Comparative effectiveness of the bivalent BA.4–5 and BA.1 mRNA-booster vaccines in the Nordic countries. medRxiv; 10.1101/2023.01.19.23284764, 19 January 2923, preprint: not peer reviewed. [DOI]
- 21. Hosseini-Moghaddam SM, He S, Calzavara A, Campitelli MA, Kwong JC.. Association of influenza vaccination with SARS-CoV-2 infection and associated hospitalization and mortality among patients aged 66 years or older. JAMA Netw Open 2022;5:e2233730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nelson JC, Jackson ML, Weiss NS, Jackson LA.. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J Clin Epidemiol 2009;62:687–94. [DOI] [PubMed] [Google Scholar]
- 23. Bar-On YM, Goldberg Y, Mandel M. et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med 2022;386:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arregocés-Castillo L, Fernández-Niño J, Rojas-Botero M. et al. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev 2022;3:e242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferdinands JM, Rao S, Dixon BE. et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ 2022;379:e072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malato J, Ribeiro RM, Fernandes E. et al. Stability of hybrid versus vaccine immunity against BA.5 infection over 8 months. Lancet Infect Dis 2023;23:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bozio CH, Butterfield KA, Briggs Hagen M. et al. Protection from COVID-19 mRNA vaccination and prior SARS-CoV-2 infection against COVID-19-associated encounters in adults during Delta and Omicron predominance. J Infect Dis 2023;227:1348–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analysed during the current study come from the national emergency preparedness registry for COVID-19 (Beredt C19), housed at the Norwegian Institute of Public Health. The preparedness registry comprises data from a variety of central health registries, national clinical registries and other national administrative registries. Legal restrictions prevent the researchers from sharing the data set used in the study. However, external researchers are freely able to request access to linked data from the same registries from outside the structure of Beredt C19, as per the normal procedure for conducting health research on registry data in Norway (https://www.helsedata.no). Further information on the preparedness registry, including access to data from each data source, is available at https://www.fhi.no/en/id/corona/coronavirus/emergency-preparedness-register-for-covid-19/. Code and model results in summary from R are available from the author.