Abstract
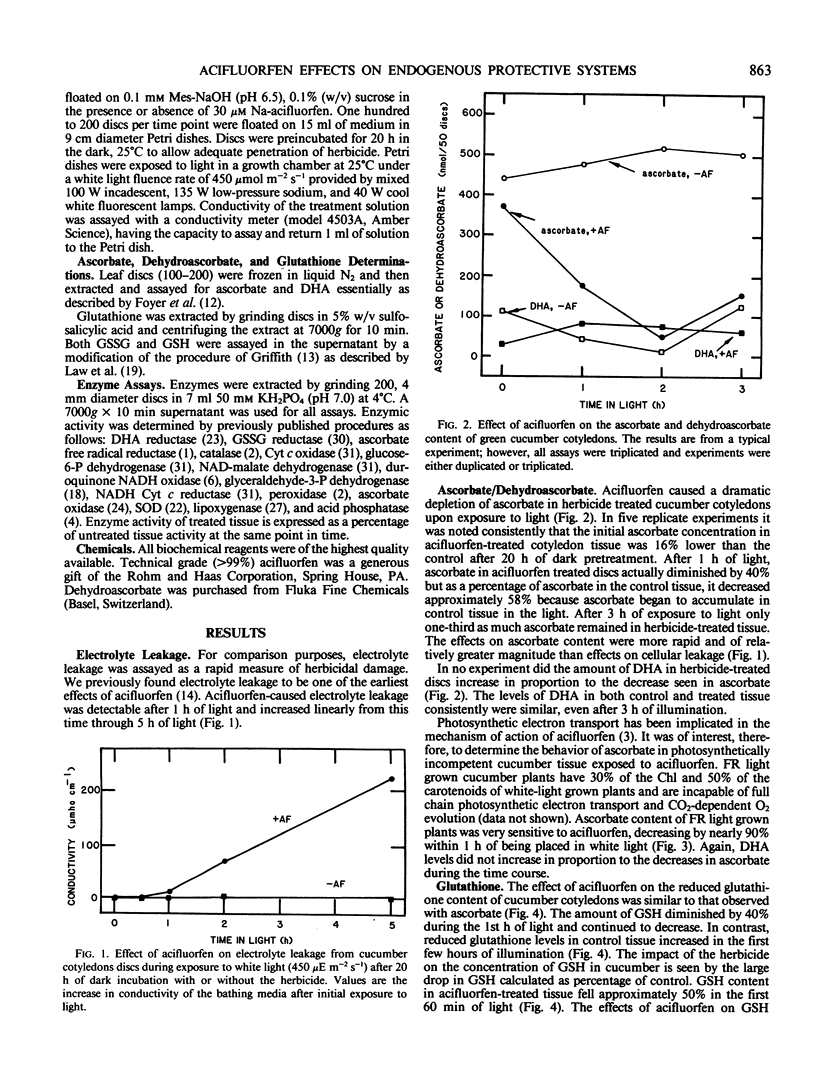
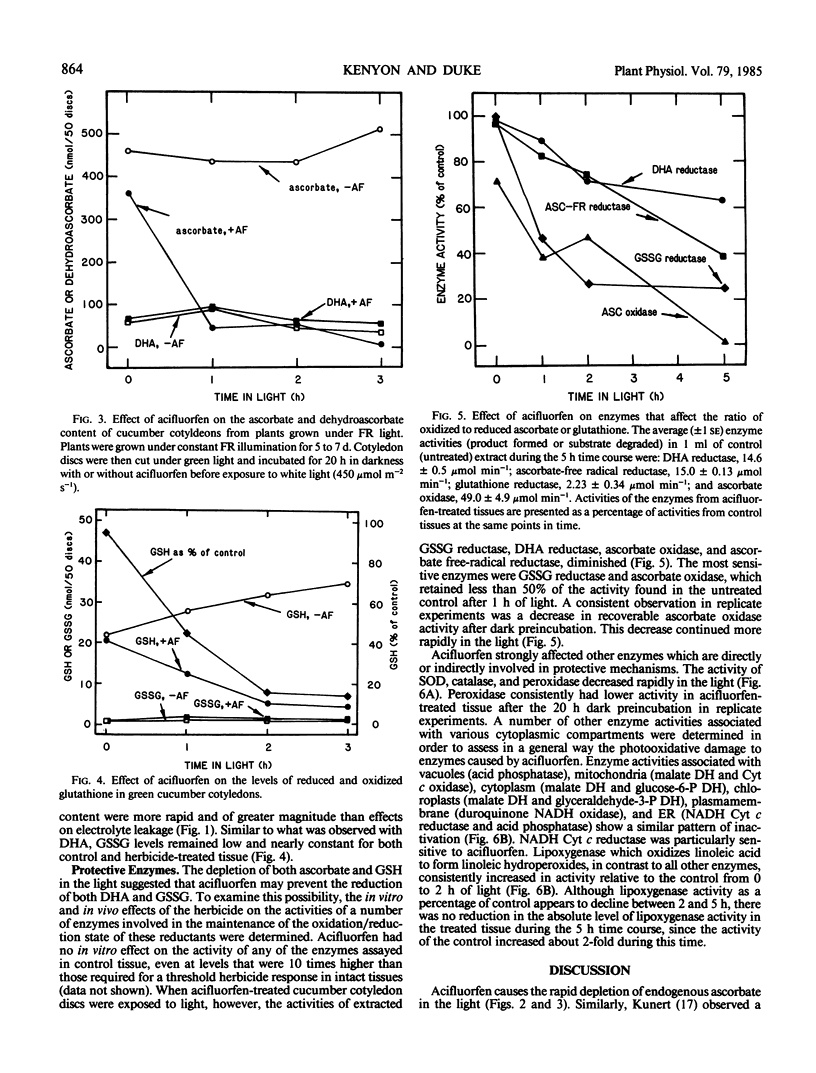
The herbicide acifluorfen (2-chloro-4-(trifluoromethyl)phenoxy-2-nitrobenzoate) causes strong photooxidative destruction of pigments and lipids in sensitive plant species. Antioxidants and oxygen radical scavengers slow the bleaching action of the herbicide. The effect of acifluorfen on glutathione and ascorbate levels in cucumber (Cucumis sativus L.) cotyledon discs was investigated to assess the relationship between herbicide activity and endogenous antioxidants. Acifluorfen decreased the levels of glutathione and ascorbate over 50% in discs exposed to less than 1.5 hours of white light (450 microeinsteins per square meter per second). Coincident increases in dehydroascorbate and glutathione disulfide were not observed. Acifluorfen also caused the rapid depletion of ascorbate in far-red light grown plants which were photosynthetically incompetent.
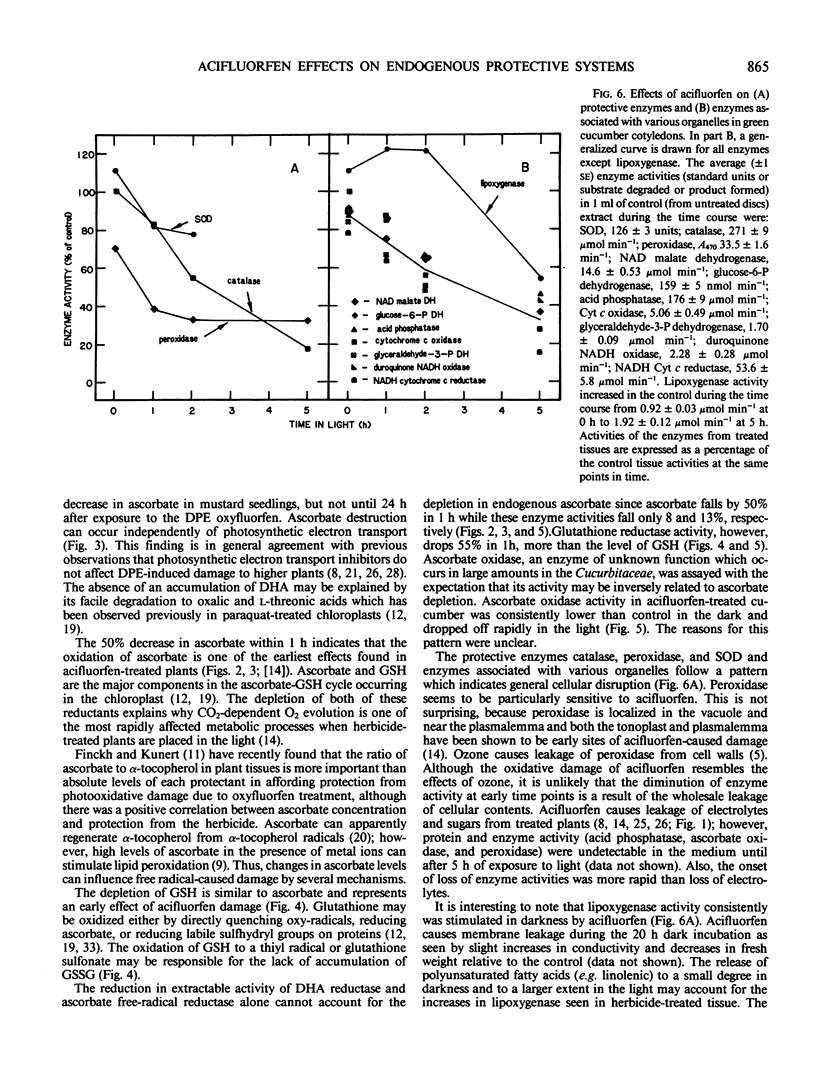
Glutathione reductase, dehydroascorbate reductase, superoxide dismutase, ascorbate oxidase, ascorbate free radical reductase, peroxidase, and catalase activities rapidly decreased in acifluorfen-treated tissue exposed to white light. None of the enzymes were inhibited in vitro by the herbicide. Acifluorfen causes irreversible photooxidative destruction of plant tissue, in part, by depleting endogenous antioxidants and inhibiting the activities of protective enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume D. E., McClure J. W. Developmental Effects of Sandoz 6706 on Activities of Enzymes of Phenolic and General Metabolism in Barley Shoots Grown in the Dark or under Low or High Intensity Light. Plant Physiol. 1980 Feb;65(2):238–244. doi: 10.1104/pp.65.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo F. J., Penel C., Greppin H. Peroxidase Release Induced by Ozone in Sedum album Leaves: Involvement of Ca. Plant Physiol. 1984 Apr;74(4):846–851. doi: 10.1104/pp.74.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumelin E. E., Tappel A. L. Hydrocarbon gases produced during in vitro peroxidation of polyunsaturated fatty acids and decomposition of preformed hydroperoxides. Lipids. 1977 Nov;12(11):894–900. doi: 10.1007/BF02533308. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. Y., Charles S. A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983 Mar 15;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H. W., Vang M. J., Mavis R. D. The cooperative interaction between vitamin E and vitamin C in suppression of peroxidation of membrane phospholipids. Biochim Biophys Acta. 1981 May 22;664(2):266–272. doi: 10.1016/0005-2760(81)90049-7. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- OBERBACHER M. F., VINES H. M. Spectrophotometric assay of ascorbic acid oxidase. Nature. 1963 Mar 23;197:1203–1204. doi: 10.1038/1971203a0. [DOI] [PubMed] [Google Scholar]
- Orr G. L., Hess F. D. Mechanism of Action of the Diphenyl Ether Herbicide Acifluorfen-Methyl in Excised Cucumber (Cucumis sativus L.) Cotyledons : LIGHT ACTIVATION AND THE SUBSEQUENT FORMATION OF LIPOPHILIC FREE RADICALS. Plant Physiol. 1982 Feb;69(2):502–507. doi: 10.1104/pp.69.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman T. K., Siedow J. N. Structural Features Required for Inhibition of Soybean Lipoxygenase-2 by Propyl Gallate : Evidence that Lipoxygenase Activity Is Distinct from the Alternative Pathway. Plant Physiol. 1983 Jan;71(1):55–58. doi: 10.1104/pp.71.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaedle M. Chloroplast glutathione reductase. Plant Physiol. 1977 May;59(5):1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers H., Sies H. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur J Biochem. 1983 Dec 1;137(1-2):29–36. doi: 10.1111/j.1432-1033.1983.tb07791.x. [DOI] [PubMed] [Google Scholar]


