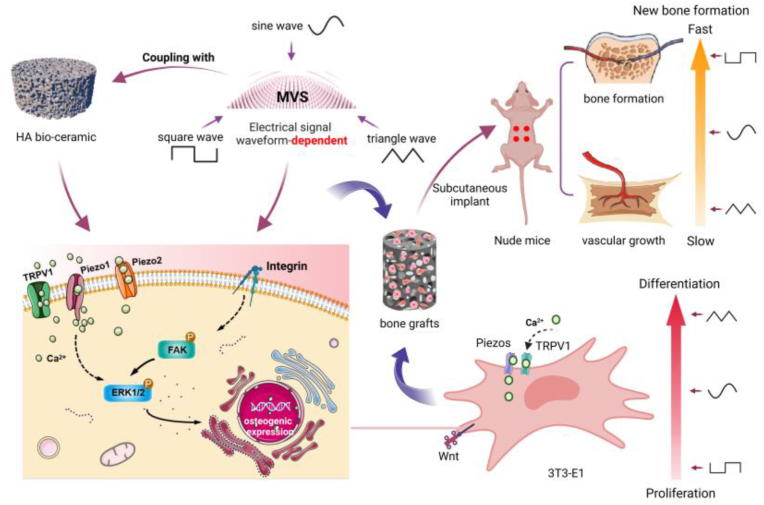
Abstract
The strategy of coupling the micro-vibration mechanical field with Ca/P ceramics to optimize the osteogenic microenvironment and enhance the functional activity of the cells can significantly improve the bone regeneration of the graft. However, the regulation mode and mechanism of this coupling strategy are not fully understood at present. This study investigated the influence of different waveforms of the electrical signals driving Microvibration Stimulation (MVS) on this coupling effect. The results showed that there were notable variances in calcium phosphate dissolution and redeposition, protein adsorption, phosphorylation of ERK1/2 and FAK signal pathways and activation of calcium channels such as TRPV1/Piezo1/Piezo2 in osteogenic microenvironment under the coupling action of hydroxyapatite (HA) ceramics and MVS driven by different electrical signal waveforms. Ultimately, these differences affected the osteogenic differentiation process of cells by a way of time-sequential regulation. Square wave-MVS coupled with HA ceramic can significantly delay the high expression time of characteristic genes (such as Runx2, Col-I and OCN) in MC3T3-E1 cells during in vitro the early, middle and late stage of differentiation, while maintain the high proliferative activity of MC3T3-E1 cells. Triangle wave signal-MVS coupled with HA ceramic promoted the osteogenic differentiation of cells in the early and late stages. Sine wave-MVS shows the effect on the process of osteogenic differentiation in the middle stage (such as the up-regulation of ALP synthesis and Col-I gene expression in the early stage of stimulation). In addition, Square wave-MVS showed the best coupling effect. The bone graft constructed under square wave-MVS formed new bone tissue and mature blood vessels only 2 weeks after subcutaneous implantation in nude mice. Our study provides a new non-invasive regulation model for precisely optimizing the osteogenic microenvironment, which can accelerate bone regeneration in bone grafts more safely, accurately and reliably.
Keywords: Electrical signal waveform, Micro-vibration, Hydroxyapatite, Osteogenic microenvironment, Tissue engineered bone
Graphical abstract
1. Introduction
Osteogenic microenvironment plays an important role in bone regeneration [1]. Studies have proved that cell microenvironment is a key determinant of cell behavior and function in development, physiology and pathophysiology [2,3]. Extracellular matrix (ECM) in cellular microenvironment is not only the structural basis of cells, but also the source of 3D biochemical and biophysical clues that trigger and regulate cell behavior. There is growing evidence that the 3D characteristics of the microenvironment are necessary for the development of many key cellular responses observed in vivo [4,5]. The optimization of osteogenic microenvironment can improve the control of 3D behavior of cells, thus promoting tissue regeneration [6]. However, the research in this field is still in its infancy. The discovery about the nature of cellular microenvironment interaction continues to update people's understanding for this field. Understanding and utilizing the role of chemistry, structure, mechanics and electrophysiology in the cellular microenvironment is still a major challenge in obtaining bone grafts with high bone regeneration activity [7,8].
As an organ in dynamic equilibrium for life, bone is continually regulated by mechanical signals [9,10]. It is pointed out that when the bone tissue is stimulated by various stresses, the bone tubule network within the bone tissue is deformed, which causes the microfluid flow in the network, so that the effector cells are stimulated by fluid shear stress. Effector cells transduce mechanical signals into biochemical signals to osteoblasts and osteoclasts through various pathways (including integrin system, receptor system, ion channels, etc.). This conversion leads to a series of responses, including energy metabolism, gene activation, secretion of growth factors, and matrix synthesis, so as to regulate the growth, resorption, remodeling, and formation of new bone tissue [11,12].
Studies have indicated that micro vibration stimulation (MVS) has a positive impact on promoting bone formation and remodeling, which is similar to the mechanical stimulation produced by muscle exercise [13,14]. Under MVS, the mechanical stimulation receptors on the surface of the cell membrane transmit mechanical signals to the cytoskeleton for recombination. On the one hand, the mechanical signals are transmitted to the nucleus through the LINC complex (linker of nucleoskeleton and cytoskeleton) [15], thus causing conformational changes in the nuclear cytoskeleton and chromatin, and directly influencing gene transcription. On the other hand, it causes changes in the activity of force-dependent ion channels or proteins, activating YAP/TAZ, Wnt, MAPK, and other signal transduction cascades, making force signal molecules into the nucleus and indirectly regulating gene expression [[16], [17], [18]]. Furthermore, MVS has been observed to significantly increase the synthesis of nitric oxide synthase (NOS) in osteoblasts and MSCs. The increased secretion of NO can promote osteogenic differentiation and vascularization, as well as regulate cell metabolism to improve the hypoxic environment.
Our previous work shows that micro-vibration coupled with BCP ceramics has the ability to change the concentration of calcium ions around cells by promoting the release of soluble calcium ions, open calcium channels on the cell membrane (such as Piezo1) to induce calcium influx, and up-regulate Runx2 signal genes by activating ERK1/2 protein to promote stem cell osteogenesis [19,20]. Simultaneously, this coupling interaction can directly stimulate the activation of Pizeo1 channels in the cell membrane and drive the influx of extracellular calcium ions. Wu et al. [21] found that the coupling of MVS and BCP ceramics can enhance the secretion of anti-inflammatory cytokines and growth factors, such as transforming growth factor-β-1 and bone morphogenetic protein-2 by activating plasma membrane Ca2+-ATPase (PMCA) channels. The BMP/TGF-Smad signaling pathway is activated through macrophage paracrine mode to induce bone marrow mesenchymal stem cells (BM-MSCs) into osteoblasts in vitro. This pathway also helps to maintain cell vitality and promote the formation of new bone tissue and blood vessels in vivo Therefore, this coupling strategy of MVS with calcium phosphate ceramics has been proved to optimize the osteogenic microenvironment and enhance cell function and activity. This strategy can enhance the in vitro vitality and in vivo survival of seed cells on the bone structure by increasing its mechanical adaptability, and thus greatly improve the bone regeneration ability of the bone graft.
However, there is still a lack of comprehensive understanding of the mechanical regulation mode and mechanism of this coupling strategy [[22], [23], [24]]. Although biological evaluation for the force field related parameters such as frequency, intensity and cycle period of MVS have been carried out, little attention has been given to the potential intervention of the electrical signal parameters that control the generation of force field on MVS, as well as the regulation effect of MVS mediated by such intervention on the biological activity of tissue-engineered bone constructs and its associated mechanism [25]. Extensive research has confirmed that electrical signal waves (sine waves, triangle waves, square waves), as direct or indirect physical and physiological stimulation parameters, have independent and important regulatory effects on the biological behavior of cells and tissues. Gabetti et al. [26] reported that electrical stimulation of different waveform pulses can significantly affect the synchronous contractile function of rat cardiac cells. Some studies found that biphasic square wave can make skin produce a higher sensory level than biphasic sine wave when studying the effect of different electrical waveform stimulation on skin sensory level [[27], [28], [29]]. Zhou et al. [30] found that the electrical signal waveform (sine wave, triangle wave and square wave) can affect the proliferation and differentiation of osteoblasts when they studied the biological activity of osteoblasts regulated by electromagnetic field in vitro. This fully shows that the electrical signal waveform is likely to be an important parameter for regulating MVS. That is, the MVS driven by the electric signal may also be interfered by the waveform parameters of the electrical signal.
In this study, based on the above MVS-calcium phosphate ceramic coupling strategy, we investigated the changes of osteoblast osteogenic differentiation and functional state in the MVS-regulated osteogenic microenvironment under the influence of electrical signal waveform and its potential mechanism. Thus, more accurate and reliable technical parameter are provided for the optimization strategy of osteogenic microenvironment by external drive force-material coupling with material. And a non-invasive and non-additive idealized model is also provided for the research of constructing implants with bone regeneration function.
2. Materials and methods
2.1. Preparation of HA materials with different surface micro-topography
Dissolve appropriate amount of anhydrous lithium chloride (Chronchem, China) and chitin (Chronchem, China) in dimethylacetamide (Chronchem, China) and stir until completely dissolved. Add HA powder (the ratio of HA/Chitin mass ratio 15:1 and 35:1, Sichuan University, China) to the sol system and stirred until uniform. Seal and stand. Pouring the slurry into the 24-hole plate, add deionized water to obtain the gelatinous HA material. Fully wash and soak to remove most of the Li2+ of the material. Drying at 37 °C for 72 h, atmospheric pressure sintering at 1200 °C (heating rate is 2.5 °C/min) to obtain HA materials with different surface microtopography (HA15, HA35). Preparation of HA porous scaffolds by template method, and he preparation procedure of HA porous scaffold is the same as that of HA15 ceramic chip paste.
2.2. Material characterization
2.2.1. Determination of micro-porosity and compressive strength
Measure the micro-porosity of HA tablets and HA scaffolds by density method. Calculate the specific surface area of HA film by Brunauer -Emmett-Teller (BET) method. Observe the surface micromorphology of the material by scanning electron microscope, and analyze the phase composition of the material by X-ray diffractometer.
Divide the porous HA scaffolds into blank group, sine wave group, triangle wave group and square wave group. Soak in PBS buffer without calcium and magnesium (Gibco, USA) according to the proportion of 20 mL•g−1, soak for 7 and 14 days at 37 °C. Rinse the sample gently with pure water and anhydrous ethanol and dry it at 150 °C for 24 h. Test compressive strength by electronic universal mechanical tester (INSTRON, USA, load rate at 0.5 mm/min, load measurement accuracy at 1 N, deformation measurement accuracy at 0.005 mm).
2.2.2. Calcium release, protein adsorption and biomimetic mineralization of HA materials
Divide the HA materials with different surface micro-topography into blank group, sine wave group, triangle wave group and square wave group. Weigh its quality in turn and soak in PBS buffer without calcium and magnesium (Gibco, USA), Serum albumin solution (Bio-Rad, USA) and SBF (Coolaber, China) according to the proportion of 20 mL/g. Put them into the micro-vibration device (self-developed, patent number CN201610900515.6), frequency at 40 Hz, magnitude at 0.3 g, and time at 30 min/24 h. Calculate the calcium release concentration per unit mass of sample (Njjcbio, China) on day 3 and 7. Calculate the protein adsorption per unit sample (BOSTER, China) on day 1 and 3. Observe the mineralized deposits on the surface of samples by scanning electron microscope (JSM 7800 F Prime, Japan) on day 3 and 7.
2.3. Biological evaluation of electrical signal waveform regulation in vitro
2.3.1. Cell culture and inoculation
MC3T3-E1 cells (the Biomaterials Engineering Research Center of Sichuan University) were cultured in α-MEM medium (GIBCO, USA) containing 10 % FCS (ExCell Bio, China). Incubate the petri dishes in a cell incubator with 5 % CO2 at 37 °C and saturated humidity.
Choose the MC3T3-E1 of P3 to P7, seeded them on the 2D plane at 2 × 104 cells/well, on the HA15 scaffolds and HA35 scaffolds at 1 × 105 cells/well. Given vibration parameter: frequency at 40 Hz, magnitude at 0.3 g, and time at 30 min/24 h, waveforms at sine wave, triangle wave and square wave. The blank group was incubated in the same environment without vibration environment.
2.3.2. Cell vitality, morphology assay and ALP protein analysis
Labeling living/dead MC3T3-E1 cells by FDA/PI double staining. Labeling of cytoskeleton proteins and nuclei by FITC (UE, China)/DAPI (Thermo fisher, USA). FDA (Thermo fisher, USA) labeled living cells turned green, while PI (Thermo fisher, USA) labeled living cells turned red. Observe by fluorescence microscope on day 1, 3 and 7. Among the high-rate images of cytoskeleton staining, select 50 cells randomly from each group on day 1, 3 and 7. Measure the angle between the cell long axis and the actin microfilament direction and the ratio of the cell long axis to the wide axis, and the data were processed and compared.
Detect cell vitality on day 1, 3 and 7 by Alamarblue working solution (Thermo fisher, USA; volume ratio of α-MEM of 10 % FCS: Alamarblue = 9:1). Add 150 μL to each well and incubated in cell incubator for 2 h. Then take 100uL samples from each well and placed in a 96-well plate, and measure optical density (OD) at 570 nm and 600 nm by enzyme labeling instrument (Bio-Rad, USA).
Detect ALP activity of cells on day 1, 3 and 7. The cells were lysed with enhanced RIPA lysate (BOSTER, China), and the protein concentration of the corresponding samples was detected by BCA kit (Thermo fisher, USA) after centrifugation. The ALP activity of cells was determined by nitrobenzene phosphate assay, and the ALP activity was normalized. The unit was expressed as NP/ug/min.
2.3.3. Extracellular matrix secretion and mineralization
MC3T3-E1 of P3 to P7 seeded on the HA15 scaffolds and HA35 scaffolds at 1 × 105 cells/well. Take out on day 3 and 7, soak 30min with 75 % alcohol after rinsing. Observe the extracellular matrix secretion and surface mineralization on two HA ceramic chips by scanning electron microscope.
2.3.4. Quantitative real-time polymerase chain reaction (RT-qPCR)
Total mRNA was extracted from four groups with RNA Simple Total RNA kit (Tiangen, China) on day 1, 3 and 7. Then transcribed by using an iScript™ cDNA synthesis kit (Bio-Rad, USA). RT-qPCR was carried out using iQTM SYBR® Green supermix (Bio-Rad, USA) and data were analyzed with the Bio-Rad CFX Manger and each RT-qPCR was performed 3 times. The related genes included Runx2, Col-I, ALP, OCN, OPN, BMP2, GAPDH, iNOs, Piezo1, Piezo2 and TRPV1. And the relative fold change of target gene in each group was calculated using the ΔΔCt method. The sequences of the primers used are listed in Table S1.
2.3.5. Western blotting
The phospho-FAK (1:800, HuaBio, China), FAK (1:5000, HuaBio, China), phospho-ERK1/2 (1:1000, HuaBio, China), ERK1/2 (1:1000, BOSTER, China), TRPV1 (1:500, ImmunoWay, USA), Piezo1 (1:500, Abcepta, China) and Piezo2 (1:1000, HuaBio, China) contents were measured on day 1, 3 and 7. MC3T3-E1 cells on 2D plane and two HA scaffolds were lysed with enhanced RIPA lysis buffer (BOSTER, China), collect supernatant at 12,000 rpm, 15 min. Then collect the supernatant, add appropriate amount of loading buffer (supernatant: loading buffer = 4:1, Biosharp, China), water bath degeneration at 95 °C for 10 min. After electrophoretic, transmembrane and sealing, first antibody incubation (4 °C, overnight incubation) and second antibody (1:2000, room temperature for 1 h, CellSignaling Technology, USA) incubation, the exposure treatment was carried out in ChemiDocTM XRS + image system (Bio-Rad, USA). The immunoblots were exposed with Image Lab software (Bio-Rad, USA). Using ImageJ software to perform semi-quantitative analysis by measuring the gray value of the bands.
2.4. Verification of functional tissue engineered bone properties by subcutaneous implantation in nude mice
2.4.1. Surgical procedure
This study was approved by the Animal feeding and Ethics Committee of Southwest Jiaotong University, all animal tests were issued by the National Academy of Sciences of China. Twenty-four Nude mice were randomly divided into four groups. Anesthetized by intraperitoneal injection of 0.1 % pentobarbital sodium (0.1 mL/20 g body weight) in a sterile environment. Cut open the central skin of the back about 1 cm wound and implant composite HA scaffolds (long at 3 mm, wide at 3 mm, high at 3 mm). Choose the MC3T3-E1 of P3 to P7, seeded them on the porous HA scaffold at 1 × 106 cells/well. Before implantation, the composite HA scaffolds coupled with electrical signals dependent on driving micro vibration stimulation for 7 days. Experimental groups included sine wave (n = 6), triangle wave (n = 6), square wave (n = 6) and bank group (n = 6). An equal number of nude mice were randomly selected from each experimental group at 2 and 5 weeks and sacrificed, and histological and immunohistochemical analyses were performed.
2.4.2. Fluorescence imaging observation in vivo
The cell membrane was stained by DIR staining (Thermo fisher, USA) before implantation was imaged in vivo at week 2 and 5 after implantation, and the cell survival status was tracked.
2.4.3. Histological and morphological examination
At 2 and 5weeks postoperatively, the sample slices of cell scaffolds implanted into nude mice were washed with PBS for 20 min three times and was fixed with 95 % ethanol. Washed and stained with hematoxylin dye (Beyotime, China). After fully washed with tap water, stained with eosin dye (Beyotime, China) 1 min. After washing and drying, neutral gum was sealed. Observe the staining under the microscope.
The sample slices of cell scaffolds implanted into nude mice for 2 and 5 weeks were fixed with formaldehyde and then dewaxed to water, washed with tap water and distilled water in turn, and then stained with hematoxylin staining solution for nuclear 5–10min. After fully washing, stained with Masson Ponceau S for 5–10 min. Soak in 2 % glacial acetic acid aqueous solution for a while, 1 % phosphomolybdic acid aqueous solution to differentiate 3–5min, and then dye 5min directly with aniline blue without washing. Soak in 0.2 % glacial acetic acid aqueous solution for a while, then seal with 95 % alcohol, anhydrous alcohol, xylene transparent, neutral gum.
2.4.4. Immunohistochemical staining
The sample slices of cell scaffolds implanted into nude mice for 2 and 5 weeks were fixed with formaldehyde and then dewaxed to water. Incubated with 3 % H2O2 at room temperature for 10 min. Rinse with distilled water and soak in PBS for 5 min. After closed, first antibody (anti-rat Col-I (1:400, Abcam, UK), anti-rat OCN (1:400, Abcam, UK), anti-rat BMP2 (1:400; Abcam, UK) and second antibody incubation (Abcam, UK), add the second generation horseradish enzyme labeled streptomycin working solution, incubate at 37 °C for 30 min, wash by PBS and use hematoxylin re-staining, sealing. Quantification data were performed by Image-J software.
2.5. Statistical analysis
The relevant data of this study are processed by GraphPadPrism8 and expressed in the form of mean ± SD. Data analysis methods include single-factor analysis of variance, two-way analysis of variance and test. All the tests in this study determined that P < 0.05 was a significant difference, which was statistically significant. All the results were reproduced in at least three separate experiments.
3. Results
3.1. Electrical signal waveforms affect calcium and phosphorus dissolution-re-deposition and protein adsorption of HA material by adjusting external force field
Two HA materials with different micromorphology were obtained by atmospheric pressure sintering at 1200 °C (heating rate of 2.5 °C/min), and the materials were white flakes. The SEM scanning results of HA15 (Fig. 1A a1, a2) show that there are a large number of micropores on the surface and the micro-porosity is (27 ± 2.1)%.Most of them are distributed in about 5 μm, and a few micropores are less than 1 μm. The SEM scanning results of HA35 (Fig. 1A a3, a4) show that the surface is smooth and dense. There are only a few micropores and the micro-porosity is 10.4 ± 3.3 %. The po-rosity of porous HA scaffolds is (85.7 ± 3.1)%, and the surface micromorphology is similar to that of HA15.
Fig. 1.
Characterization of basic properties of materials. (A) Surface Microtopography of two kinds of ceramic sheets HA15 (a1, a2) and HA35 (a3, a4); (B) XRD composition analysis of HA15 and HA35;(C) Compressive strength of the porous HA scaffold; (D) Ca2+ release of HA15 and HA35;(E) Protein adsorption of HA15 and HA35.
The XRD results of sintered HA slices (Fig. 1B) show that the spectra of the two samples sintered at 1200 °C are in good agreement with the standard ones (JCPDS09-0432). The obvious characteristic peaks of HA appear at about 26°, 32°, 40°, 47° and 50°, and no other miscellaneous phases are found. The energy spec-trum results also show that no other substances have been introduced. The compression test results (Fig. 1C) showed that the compressive strength of the porous scaffold did not change compared with the initial strength after soaking in the micro-vibration environment of sine wave, triangle wave and square wave for 7 and 14 days. The micro-vibration of three different electrical signal waveforms does not significantly affect the compressive strength of two kinds of HA porous materials.
The results of calcium release (Fig. 1D) showed that the calcium release in each experimental group was significantly higher than that in the control group, and that in the HA15 group was significantly higher than that in the HA35 group. The calcium release of the sine group was the highest on the 3rd day, the triangle wave group was the highest on the 7th day, and the lowest in the square wave group. The results of protein adsorption (Fig. 1E) showed that the amount of protein adsorption per unit mass of each group increased with time, and the experimental groups were significantly higher than the control group, and the HA15 groups were significantly higher than that the HA35 groups. Among the experimental groups, the triangular wave showed the strongest adsorption, while the square wave showed the weakest.
The SEM results of mineralization in HA15 group (Fig. S1) showed that after 3 days of mineralization, a small amount of mineralized sediments showed loose floc sporadic distribution. The amount of mineralized sediments in sine wave group and triangle wave group is slightly more than that in static group and square wave group. After 7 days of mineralization, the number of mineralized sediments in each group increased significantly, and the morphology of mineralized sediments in each group was signifi-cantly different, such as irregular conical and flocculent deposits in the static group, wire mesh in the sine wave group, uniform and dense flower clusters in the triangular wave group, and granule accumulation in the square wave group. However, in the HA35 group (Fig. S1), only few of minerals was formed in each group after 3 days of mineralization (the red triangles in the picture), which in the experimental group was slightly more than that in the control group, and there was no obvious change in mineralization morphology after 7 days of mineralization.
3.2. Electrical signal waveforms affect cell activity, morphology, proliferation and osteogenic differentiation by adjusting external force field
The results of cell dead and alive staining showed (Fig. 2A) that a few dead cells were present in all groups after the first day of force stimulation. After the third day of force stimulation, the number of dead cells in each experimental group increased sig-nificantly compared to the static control group. After 7 days of force stimulation, the number of live cells in each experimental group increased significantly compared to the control group, while the number of dead cells decreased significantly. The square wave group showed obviously enhanced cell proliferation ability. The number of cell death was significantly more on the third day than on the first and seventh days.
Fig. 2.
(A)Live/dead of MC3T3-E1 cells grown on 2D plane on day 1、3 and 7. Scale bar = 400 μm; (B)Cell activity of MC3T3-E1 on 2D plane for different waveforms on day1, 3 and 7; (C)ALP of MC3T3-E1 on 2D plane for different waveforms on day 3 and 7; (D-F)Analysis of angle between cell length-width-axis and analysis of cell skeleton; (G)mRNA expression of osteogenesis-related genes (Runx2, Col-I, iNOs, ALP, OPN, OCN) of MC3T3-E1 on 2D plane after implanted in vivo for different waveforms on day1, 3 and 7; (H) Phosphorylation of FAK and ERK1/2 of MC3T3-E1 on 2D plane for different waveforms on day 1, 3 and 7 and semi-quantitative analysis with software ImageJ, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Compared with the control group (Fig. S3), each experimental group upregulated the expression of cytoskeleton protein F-actin at the initial stage of force addition, and continued to increase synthesis with time. F-actin expression was most significantly upregulated in the triangular wave electrical signal group. The fluorescence intensity of the square wave group reached the strongest after 7 days of the acceleration.
Cell proliferation (Fig. 2B) showed that the number of cells in each group in-creased significantly with the passage of time, and the square wave group showed the strongest trend of promoting cell proliferation. Alkaline phosphatase (ALP) activity is detected as shown in (Fig. 2C). Triangle wave group had the highest activity of pro-ALP expression, and the expression of ALP in each experimental group was sig-nificantly higher than that in the static group, and reached the highest expression on the third day. The square wave group was significantly lower than the other three groups.
Cytoskeleton plays a key “bridge” role in transferring extracellular mechanical signals into cells. Analyze the ratio of length to width axis and the angle between cell major axis and actin microfilament (F-actin) in each group (Fig. 2D). The ratio of cell length to width of axis in each group was lower than that in the control group, and the ratio in the triangular wave group was the lowest. The Angle analysis of the long axis of the cell and actin microfilaments showed an increasing trend in all experimental groups, and the triangle wave > square wave > sine wave. (Fig. 2E、F).
The expression levels of osteogenic marker genes of 2D surface cells on the 1, 3 and 7 days are shown in Fig. 2G. The control group showed almost no change in gene expression levels, but there was a different trend in the stress group. In the early stage of mechanical stimulation, the expression of Col-I in square wave group (Fig. 2G) was significantly higher than other groups. The gene expression trend of Runx2 (Fig. 2G) and ALP (Fig. 2G) in each experimental group have the same trend. On the third day, the expression level of Runx2 gene in triangular wave group was significantly higher than other groups and followed by square wave, which indicating that triangular wave up-regulated cell differentiation ability and square wave upregulated cell prolix-eration ability. However, in the middle and late stage of stress, the gene expression level of OCN (Fig. 2G) in sine wave group was significantly higher than that in square wave group and triangle wave group.
3.3. Regulation of cellular ERK1/2 and FAK phosphorylation by MVS force field under the intervention of electrical signal waveforms
WB results (Fig. 2H) showed that the ratio of ERK phosphorylation in the sine wave group was higher than the other three groups on the first day, and on the third day of force, the ratio of ERK phosphorylation in all three MV groups was significantly higher than the blank group. On the seventh day, the phosphorylation ratio decreased in the square wave group and triangle wave group, and was almost the same in the si-ne wave group and the static group.
The results of FAK showed that all the four groups exhibited a phosphorylation trend of increasing at first and then decreasing, with the most obvious change in the square wave group and the weakest in the triangle wave group. Among them, the phosphorylation in the square wave group was the most significant on the first day and the third day, and the phosphorylation of the sine wave group reached the peak on the third day, which remained significantly better than other three groups.
3.4. Activity, morphology, proliferation and osteogenic differentiation of tissue engineering bone
After three days of composite culture of HA materials and cells, cells spread and grew on the surface of the HA15 material, and each experimental group showed more extracellular matrix secretion (Fig. 3C and G). After 7 days, the cells of the experi-mental groups on HA15 showed a tendency to grow in cascades and secreted a large amount of extracellular matrix. More granular mineralized sediments were observed, while the groups of cells on HA35 showed more obvious mineralized sediments (Fig. 3D and H). The proliferation results of each group (Fig. S4) showed that the proliferative activity of cells on the two constructs showed the same trend. The proliferative activity in the triangular wave and square wave groups was lower than the static group, and in the late, the experimental groups were higher than the static group. The prolifera-tive activity of the square wave group was significantly higher than that of the static group, and the difference was more significant between the square wave group and the static group at the later stage of force addition.
Fig. 3.
(A)Live/dead of MC3T3-E1 cells grown on HA15 for different waveforms on day 1 、 3 and 7. Scale bar = 400 μm; (B) F-actin staining of MC3T3-E1 cells grown on HA15 for different waveforms on day 1、3 and 7. Scale bar = 400 μm; (C) Mineralization of extracellular matrix of MC3T3-E1 cells grown on HA15 for different waveforms on day 3; (D) Mineralization of extracellular matrix of MC3T3-E1 cells grown on HA15 for different waveforms on day 7; (E)Live/dead of MC3T3-E1 cells grown on HA35 for different waveforms on day 1、3 and 7. Scale bar = 400 μm. (F) F-actin staining of MC3T3-E1 cells grown on HA35 for different waveforms on day 1、3 and 7. Scale bar = 400 μm. (G) Mineralization of extracellular matrix of MC3T3-E1 cells grown on HA35 for different waveforms on day 3; (H) Mineralization of extracellular matrix of MC3T3-E1 cells grown on HA35 for different waveforms on day 7. Blue squares represent extracellular matrix secretion; red triangles represent mineralization; yellow circles represent cells.
The results of ALP activity showed (Fig. S4) that the triangle wave group had the highest ALP-promoting activity expression. On the third day, the ALP activity in the experimental groups was higher than the control group, and ALP activity in the HA35 construct group was significantly higher than the blank group. On the seventh day, ALP activity in each experimental group was significantly decreased. The results ob-served under fluorescence microscope (Fig. 3A and E) showed that the trend of cell death and survival in static group and experimental group was the same as that in two-dimensional culture. The cell proliferation in the HA15 groups (Fig. S4) were significantly better than that in the HA35 (Fig. S4) group on the seventh day. Materials driven by different waveform electrical signals coupled with MVS had a modulating effect on the cell dead or alive status. The results of cytoskeleton staining in each group (Fig. 3B and F) showed that in the HA15 groups (Fig. 3B), the synthesis of F-actin protein in experimental groups was significantly higher than that in the control group after 7 days of force, especially the sine and triangle waves. In the HA35 groups (Fig. 3E), the synthesis of F-actin protein in the sine wave group was significantly higher than that in the other groups after 7 days of stress.
For different electrical signal waveforms, the gene expression level of static group did not change much, and the expression level of osteogenic gene in the HA15 material groups (Fig. 4A) was significantly higher than that HA35 material (Fig. 4B). In the early stage of stress, the expression of Runx2, BMP2, OCN and OPN genes in triangular wave group of HA15 material was significantly higher than that in the other three groups (Fig. 4A), but in the middle stage of stress, the expression level of osteogenic genes in triangular wave group and square wave group was downregulated, while that in sine wave group was increased. Then in late, square wave group was increased. The osteogenic genes expression trends of cells in the triangular wave group on HA35 material (Fig. 4B) were similar to the HA15 group, but the square wave group and sine wave group were different. In the early stage of MVS, Col-I and OPN in triangle wave group were significantly better than those in other groups, but in the middle stage, the gene expression levels of Runx2 and Col-I in square wave group and sine wave group were significantly higher than triangle and static group. In the late stage, the gene expression levels were further up-regulated in sine wave group, and the gene expression levels of BMP2, Runx2 and OCN in sine wave group were significantly higher than other groups.
Fig. 4.
(A)mRNA expression of osteogenesis-related genes(Runx2, BMP2, Col-I, OPN and OCN)of MC3T3-E1 grown on HA15 for different waveforms on day 1、3 and 7. (B)mRNA expression of cation channel genes(iNOs, Piezo1, Piezo2 and TRPV1)of MC3T3-E1 grown on HA15 for different waveforms on day 1、3 and 7. (C) mRNA expression of osteogenesis-related genes(Runx2, BMP2, Col-I, OPN and OCN)of MC3T3-E1 grown on HA35 for different waveforms on day 1、3 and 7. (D) mRNA expression of cation channel genes (iNOs, Piezo1, Piezo2 and TRPV1) of MC3T3-E1 grown on HA15 for different waveforms on day 1、3 and 7.
3.5. Molecular signaling pathway mechanism of osteogenic differentiation regulated by different electrical signal waveforms which control external MVS and couple with HA bio-ceramic
On the first day of stress, the gene expression of cells in the triangle wave group on HA15 scaffold (Fig. 4C) reached the peak level, which was significantly higher than the other two groups. In the middle and late stage of stress, the gene expression level of triangle wave group and square wave group decreased gradually, while the sine wave group and static group increased at first and then decreased. On the seventh day, the gene expression level of each group was lower. For the cells on the HA35 scaffold (Fig. 4D), the square wave group and the triangle wave group had the same trend in the early stage of stress, and reached the peak on the third day, which was significantly higher than the sine wave group and static group. The gene expression level in the sine wave group decreased at first and then increased, and on the seventh day, the expression level was significantly higher than other three groups. The results of Piezo1 expression (Fig. 4C) showed that the cells of the sine wave group on the HA15 scaffold maintained a high level of expression, and the expression level in the triangle wave group decreased significantly on the third day. On the seventh day, there were significant differences between the three experimental groups and the static group. The results of Piezo2 gene expression (Fig. 4C) showed that the trend of gene expression in the sine wave group on the HA15 scaffold reached the peak on the third day, which was similar to that of TRPV1, the gene expression level in the triangle wave group remained high level, the square wave group decreased at first and then increased, and the static group remained at a low level. On the seventh day after stress, on the HA35 scaffold (Fig. 4D), the gene expression level in the triangle wave group increased significantly, followed by the square wave group, while the gene expression level in the sine wave group and static group showed a lower trend.
WB results (Fig. 5, Fig. 6) showed that the phosphorylation levels of ERK and FAK in the sine wave group were significantly higher than those in the other three groups on the first day. On the third day, the phosphorylation levels of ERK and FAK in the square wave group were continuously up-regulated. On the seventh day, the phosphorylation levels of ERK in the square wave group and the triangle wave group were significantly down-regulation, but the phosphorylation levels of FAK in the square wave group continued to increase (Fig. 5C–F). Compared to the static group, the three electrical signal waveforms groups activated both Piezo1 and Piezo2 channels in the early stage (at the first day), but only the square wave group on the HA15 ceramic activated TRPV1 channel on the first day. On the third day, Piezo1, Piezo2, and TRPV1 on three electrical signal waveform groups were all enhanced activated. On the seventh day, this up-regulated activation level generally decreased, except for the sine wave group (Fig. 6).
Fig. 5.
(A) Western blot results of phosphorylation of ERK1/2 of MC3T3-E1 grown on two scaffolds for different waveforms on day 1, 3 and 7. (B) Western blot results of phosphorylation of FAK of MC3T3-E1 grown on two scaffolds for different waveforms on day 1, 3 and 7. (C–D) Semi-quantitative analysis of phosphorylation of ERK1/2 of MC3T3-E1 grown on two scaffolds for different waveforms on day 1, 3 and 7 with software ImageJ, n = 6. (E–F) Semi-quantitative analysis of phosphorylation of FAK of MC3T3-E1 grown on two scaffolds for different waveforms on day 1, 3 and 7 with software ImageJ, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Fig. 6.
(A) Western blot results of phosphorylation of Piezo1, Piezo2 and TRPV1 of MC3T3-E1 grown on two scaffolds for different waveforms on day 1, 3 and 7. (B) The relative protein (Piezo1, Piezo2 and TRPV1) expression levels of cells grown on HA35 and HA15 coupled with different waveforms on day 1, 3 and 7 measured by using software ImageJ.n = 6, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
3.6. Maintenance of activity of bone graft regulated by electrical signal waveform in subcutaneous implantation model of nude mice
Fluorescence imaging of living cells (Fig. 7) showed that for the same time points, there were differences in fluorescence intensity between the four groups within 2 weeks after implantation. The fluorescence intensity of grafts in sine wave group and square wave group was significantly brighter than that in static control group and triangle wave group. With the passage of time, the fluorescence intensity of the grafts in the experimental groups was more significant than that in the control group. In terms of the time scale change of fluorescence intensity, the fluorescence intensity in the central area of all experimental groups tended to reach the maximum at 2 weeks after implantation, and did not decrease significantly with time. The fluorescence in-tensity in the central area of the static control group tended to reach the maximum within 2 weeks, but then attenuated obviously within 5 weeks of implantation. Among the three experimental groups, the fluorescence intensity of triangle wave group is the weakest, while the square wave group is the strongest, and the sine wave group is in the middle.
Fig. 7.
(A) Detailed process of subcutaneous implantation for tissue engineered bone grafts; (B) In vivo fluorescence results of subcutaneous implantation in Blank, Square, sine and triangle wave group with MVS condition after implantation for 1, 2 and 3 weeks; (C) In vivo fluorescence results semi-quantification (D)Immunohistochemical semi-quantification of BMP2; (E)Immunohistochemical semi-quantification of Col-I; (F)Immunohistochemical semi-quantification of OCN(I) in Blank, Square, Sine and Triangle wave group for subcutaneous implantation with MVS condition after implantation for 2 and 5 weeks.
3.7. Histology observation of new bone formation and vascularization after subcutaneous implantation of functional tissue engineered bone
The results of HE staining (Fig. 8A) showed that after 2weeks of subcutaneous implantation, the degree of new bone/osteoid formation was in the order of square wave group > sine wave group > triangle wave group > static group. New bone and osteoid in the square wave group were evenly distributed in the scaffold, while the triangle wave group and sine wave group were mainly distributed in the peri-implantation pore area. However, in the static control group, only a few sporadic callus formed and distributed in the periporeal pores. 5 weeks after implantation, a large number of new bone and osteoid were formed in all MVS groups, and mineralized and mature bone tissues appeared in the square wave group. New bone and osteoid in square wave group and square wave group showed a tendency of uniform distribution in the scaffold, while triangular wave group and static group were still concentrated in the peripheral pore area of the implant.
Fig. 8.
(A) Histological analysis of subcutaneous implantation in Blank, Square, Sine and Triangle wave group with MVS condition after implantation for 2 and 5 weeks. (B) Masson's analysis of subcutaneous implantation in Blank, Square, Sine and Triangle wave group with MVS condition after implantation for 2 and 5 weeks. (C) Semi-quantitative results of in vivo fluores-cence after subcutaneous implantation with MVS condition for 2 and 5 weeks. (D) Semi-quantitative results of new bone area after subcutaneous implantation with MVS condition for 2 and 5 weeks. (E) Semi-quantitative results of Vascular growth degree after subcutaneous im-plantation with MVS condition for 2 and 5 weeks. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Masson's trichromatic staining results (Fig. 8B) showed that after 2 weeks of implantation, the new bone mineralization maturity of square wave group and triangle wave group was higher than that of sine-wave group and control group. The results showed that the square wave group was the highest, the sine wave group and the triangle wave group were higher than the static group. The content of type I collagen in new bone tissue was in the order of square wave group > triangle wave group > sine wave group > static group. After 5 weeks of implantation, the mineralization maturity of new bone was in the order of square wave group > triangle wave group > sine wave group > static group. Obvious new bone formation and neovascularization were observed in the implants of all experimental groups, and the trend of neovascularization was similar to that at 2 weeks. Type I collagen content in new bone tissue was in the order of square wave group > sine wave group > triangle wave group > static group, which was consistent with the trend of new bone formation.
The results of immunohistochemistry (Fig. 9A) showed that After 2 weeks of im-plantation, the expression of BMP2 (Fig. 9B) was the highest in the triangular wave group, and higher in the square wave group and the sine wave group than in the static group. However, the expressions of Col-I (Fig. 9C) and OCN (Fig. 9D) were square wave group > sine wave group > triangle wave group > static group. After 5 weeks of implantation, the expression of BMP2 was significantly down-regulated in all experimental groups, the most significantly down-regulated in the Chinese wave group and the triangular wave group, while significantly up-regulated in the control group. The expression of Col-I was the highest in the square wave group, and higher in the sine wave and triangle wave groups than in the static group. The expres-sion of OCN was triangle wave group > square wave group > sine wave group > static group.
Fig. 9.
(A)Immunohistochemical images and quantification of BMP2 (B),Col-I (C) and OCN (D) in Blank, Square, Sine and Triangle wave group for subcutaneous implantation with MVS condition after implantation for 2 and 5 weeks. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
4. Discussion
Large segmental bone defect has always been a key and difficult problem in clinical treatment, and the existing technical methods cannot solve it [31,32]. Autogenous bone transplantation has the disadvantage of limited bone mass, while allogeneic bone transplantation faced some problems such as immune rejection, complications and even infection. Therefore, bone tissue engineering emerged with its needs. However, bone is a kind of mechanical load organs, which is always subjected to changing forces and in a constantly changing force field, and the development and reconstruction of which are carried out under the environment of mechanical load [33]. Therefore, how to make the bone graft adapt to the physiological/mechanical environment of the host bone as soon as possible, to effectively promote bone regeneration, and to design and reconstruct ideal osteogenic microenvironment in vitro are of great significance.
Studies have shown that micro-vibration (MVS, low amplitude, low magnitude, low frequency mechanical stimulation with amplitude ≤50 μ m, intensity <1×g, frequency range at 1 Hz–100 Hz) is a physiological adaptive mechanical stimulation, which can inhibit bone resorption, promote bone formation and reconstruct MVS. MVS can cause the two-way flow of liquid, thus stimulating the fluid flow in the tubular network under physiological conditions in the body. In addition, the researchers found that micro-vibration can stimulate osteocytes to produce soluble factors that inhibit osteoclast formation and regulate the osteogenic differentiation and mineralization of BMSCs [34]. In these studies, as a mechanical load similar to physiological stimulation, MVS is considered to be the most easily used mechanical stimulation to apply in vivo. The bi-directional fluid flow induced by MVS can simulate the fluid state felt by osteocytes in the bone tubule network under the condition of physiological movement in vivo. Clinical and basic research have demonstrated that MVS can reduce bone resorption by inhibiting osteoclast resorption but promotes osteogenic differentiation, thereby enhancing bone formation and bone remodeling [35]. At the same time, as a non-invasive biophysical stimulation, the anabolism of MV at tissue and cellular level has been confirmed. Chow et al. found that MVS with low amplitude and high frequency can stimulate the immune response at the fracture site, regulate macrophage polarization, and significantly improve the delay of bone healing caused by ovariectomy [36].
It is also found that the physical and chemical properties of bio-ceramic scaffolds and their concave or geometric shape provide a necessary osteogenic microenvironment for osteogenesis and bone formation [37]. The participation of calcium ions can promote cell migration and differentiation, thus accelerating the formation of blood vessels and new bone at the defect site. Pore structure that can affect angiogenesis is one of the prerequisites for tissue formation induced by bioceramic scaffolds. In addition, angiogenesis has been proved to promote osteogenesis together with bioceramic [38]. Therefore, bioceramics with high porosity, high permeability and good osteogenic induction can provide a good osteoinductive microenvironment for tissue engineering bone grafts.
Our previous studies have shown that appropriate MVS (intensity at 0.3 g, frequency at 40 Hz and 30 min/24 h) can not only promote the proliferation, adhesion and osteogenic differentiation of BMSCS and osteoblasts, but also enhance their mechanical adaptability and cell activity [20,39]. In addition, our previous work also confirmed that MVS with the same parameters can promote calcium release, protein adsorption and mineralization deposition of calcium phosphate bioceramic scaffolds [40,41]. The dynamic dissolution and release of calcium ions in bioceramic scaffolds, protein adsorption and biomimetic mineralization are the key factors for osteogenic micro-environment constructed by calcium phosphate materials. These findings show that MVS can be coupled with bioceramic scaffolds to provide an optimized osteogenic microenvironment for cells, thus improving the bone regeneration activity of the grafts.
Although we have proved that using this strategy to enhance bone regeneration is effective, we still know little about the precise regulation and mechanical conduction mechanism of how MVS can couple to optimize the osteogenic microenvironment and cell function. We have previously reported the important role of FAK-ERK1/2 signal pathway and piezo1 calcium channel activation in the functional acquisition of bone grafts constructed by MVS coupled with BCP ceramics [20].
Therefore, in this study, we assume that the micro-vibration field driven by the electric field may be interfered by the waveform parameters of the electrical signal, which will play a fine role in regulating the osteogenic microenvironment and related cellular signals. A key goal of this study is to provide a more accurate and reliable technical parameter basis for the optimization of osteogenic microenvironment strategy by external driving force field-material coupling interaction, so as to further improve the bone regeneration function of the bone graft constructed by this strategy.
Electrical signal waveforms-driving MVS has shown obvious regulatory differences on calcium release, protein adsorption and mineralization deposition behavior of HA ceramics. The MV dynamic environment driven by triangle wave has the highest ability to promote calcium ion release and protein adsorption, while square wave is more beneficial to promote material dissolution-redeposition. Driven by different waveforms, the morphology of biomimetic mineralized deposits on the material surface is also different. This suggests that the MVS driven by different electrical signal waveforms gives different fluid shear stress to the material, and affects the protein adsorption and mineralization deposition of the material by affecting the release of calcium ions, thus obtaining the ability to finely regulate the osteogenic microenvironment. In bioceramic scaffolds, the dynamic dissolution and release of Ca2+, protein adsorption and biomimetic mineralization are the key factors for osteogenic micro-environment constructed by calcium phosphate materials. For example, the increased Ca2+ release can induce BMSCs recruitment and stimulate osteogenic differentiation of BMSCs in a concentration-dependent manner [42]. In addition, for bone tissue engineering, the surface morphology of bone biomaterial scaffolds is of great significance for cell growth, bone conduction, host reaction and tissue regeneration. Some studies have shown that the micropores on the wall surface of calcium phosphate ceramics are not only conducive to liquid flow and infiltration, accelerate the flow of liquid in the surrounding environment, but also reduce the fluid shear stress imposed by the liquid on the ceramic surface [43,44]. In this study, the change of osteogenic microenvironment of HA bioceramics with different surface microtopography coupled with MVS is consistent, but the regulation effect of HA ceramics with higher microporosity is amplified.
The apoptosis, proliferation, morphology and differentiation of osteocytes are the adaptive response of bone to the change of mechanical load. Therefore, obtaining cells with mechanical tolerance and stronger proliferative activity and differentiation ability is of positive significance to enhance the regeneration and repair function of tissue engineered bone graft [40]. The MVS driven by three kinds of electrical signal waveforms all improved the biological activity of cells, induced the early apoptosis of cells that could not tolerate mechanical stimulation, and promoted the synthesis of cytoskeleton F-actin protein, which was consistent with the results of our previous research. On this basis, the MVS driven by square wave significantly promoted cell proliferation and inhibited cell osteogenic differentiation to a certain extent. On the other hand, the triangle wave signal showed a significantly up-regulated ALP expression and an enhanced tendency to promote osteogenic differentiation than others. Additionally, the cytoskeleton reshapes its structure and tissue, thus altering the transmission of mechanical signals related to cell function [45]. Du et al. [46] have found that mechanical stretching promotes the rearrangement of cytoskeleton and reduces the cell roundness, nuclear area, cell density and cell viability. Lee et al. [45] applied compressive mechanical stress to epithelial cells. Immunostaining images of cytoskeleton showed that actin filaments were reorganized in the presence of compressive stress, the area of single cell decreased from 409 ± 55μm2 to 348 ± 23μm2 and the amount of actin in cells decreased from 66 ± 7 % to 51 ± 6 %. The results of this study showed that MVS enhanced the lateral adhesion and spreading of cells, in which the MVS driven by triangle wave made the cells have the smallest aspect-width axis ratio and the maximum cytoskeleton angle, which also suggested that MVS driven by triangle wave was the most favorable for cell osteogenic differentiation. It can thus be seen that the electrical signal waveform which driving MVS dominates the bone differentiation and proliferation ability of two-dimensional cultured osteoblasts.
What is interesting is the effect of three kinds of electrical signal waveforms on the phosphorylation of FAK-ERK1/2 signal axis. In the square wave group, the level of FAK phosphorylation was significantly up-regulated in the early stage of stress (one day later), reached the highest on the third day, and decreased in the late stage of stress; the phosphorylation activation of the sine wave group appeared in the middle stage of the stress (three days later) and maintained to the seventh day; the triangle wave group always showed a state of phosphorylation inhibition. At the same time, the ERK1/2 phosphorylation in the square wave group and triangle wave group were significantly up-regulated on the third day, and then decreased, while the sine wave group maintained a high expression of phosphorylation from beginning to end. These results suggest that MVS driven by electrical signal waveforms transmit signals with different regulatory functions to osteoblasts through FAK-ERK1/2 signals, which may also involve a more complex mechanical signal transduction network.
Then we discussed the effects of biological activity of osteoblasts caused by electrical signal waveforms-driving MVS coupled with HA ceramics. The experimental results are similar to those of two-dimensional cultured cells. The MVS driven by sine wave and triangle wave significantly up-regulated the expression of cytoskeleton F-actin and extracellular matrix mineralization after coupling with HA material, while the square wave group significantly promoted cell proliferation. Compared with HA ceramics with lower porosity, HA ceramics with higher microporosity amplify this difference. Different surface micro-morphologies affect the adsorption capacity of proteins, while the concentration of local proteins affects the binding of cells to the material surface and promotes the faster adhesion and diffusion of cells [47]. Therefore, the intensity of the interaction between cell and calcium phosphate ceramic surface further affects the physiological state of cells under MVS, it also proves that the material properties of different surface microtopography play an important role in extracellular matrix secretion and mineralization.
The results of RT-qPCR showed that the expressions of Runx2, Col-I, OCN and OPN were significantly up-regulated in the triangle wave group at the early stage of stress. The expression of square wave signal group was up-regulated in the middle and late stage, while that of sine wave group was mainly up-regulated in the middle stage. This suggests that electrical signal waveforms caused a time-sequential effect for the MVS-material coupling synergistic effect. The reason may be that different electrical signal waveforms-driven MVS act on HA materials, so that cells on different groups of HA materials are subjected to different fluid shear stress in liquid environment. On the one hand, different fluid shear stress applied to HA materials, which increases the concentration of soluble calcium ions around the cells affects the osteogenic differentiation of cells through the ion microenvironment around the cells. On the other hand, different stress stimuli regulate and activate calcium channels including TRPV1 and Piezo1/2, and then regulate the process of osteogenic differentiation. Some studies have shown that low-frequency vibration and low-level shear stress promote the phosphorylation of FAK-ERK [34,48]. Extracellular matrix-integrin interaction stimulates FAK phosphorylation, and then activates (phosphorylates) the key signal protein ERK1/2, which mediates osteogenic differentiation.
With the process of bone differentiation, the gene expression of TRPV1 and Piezo1/2 is also up-regulated. The number of ion channel expression and enhanced channel expression is related to the electrical signal waveforms and the surface micromorphology of the material. On the HA scaffolds with high microporosity, MVS driven by three waveforms upregulated the gene expression of TRPV1 and Piezo1/2 cationic channels in the early stage (the first day). However, in the middle stage (the third day), the upregulation of TRPV1 and Piezo1/2 were only seen in the sine wave-driving MVS, while triangle wave-driving MVS only upregulated Piezo2. And in the late stage (day 7), square wave-driving MVS upregulated the gene expression of TRPV1 and Piezo1/2, triangle group upregulated Piezo1/2, and sine group upregulated Piezo1. On the HA scaffold with a dense and smooth surface, such upregulation is significantly delayed. TRP cationic channels are usually associated with temperature and pain perception, and it can be activated by inflammatory mediators such as NO19 (we evaluated indirectly through the gene expression of iNOS in this work). TRP and piezo are also related to vibration mechanical transduction, Cytoskeleton and YES-related proteins (YAP, a known mechanical regulator of osteogenesis) [49]. Piezo1 and Piezo2 are new mechanically sensitive ion channels in mammals [50]. Piezo1 enables cells to sense various “outside-in” and “inside-out” mechanical forces, including radial pressure, membrane tension, compression, shear stress, matrix stiffness, ultrasound, etc., and is considered to be the main mechanical sensor in bone homeostasis [51]. Dolgorukova et al. [52] have shown that Piezo1 channels regulate physiological function by activating TRPV1. Piezo2 plays a key role in tactile sensation and is highly expressed in osteocytes. Together with Piezo1, it promotes the influx of Ca2+ induced by mechanical force, thus completing the signal transduction of mechanical force. Zhu et al. pointed out that Piezo1 channel induces BMP-2 expression through ERK1/2 and p38-MAPK signal transduction [53].
WB results showed that MVS significantly increased the phosphorylated expression of ERK1/2 signal and FAK signal, and showed a significant difference in different electrical signal waveform groups. The three experimental groups continuously upregulated the expression of ERK1/2 signal and FAK signal from the early stage, but the peak expression period was slightly different. This may be attributed to the difference between direct effects (MV) and indirect effects (activating TRPV1 and Piezo1/2 channels). In addition, the surface micromorphology of HA ceramics also affected the expression of ERK1/2 signal and FAK signal. The ceramic materials with lower microporosity significantly weakened the trend of signal expression. This may be directly related to the concentration of Ca2+ in the osteogenic microenvironment. In the coupling effect of MVS-HA ceramics, the ceramics with high microporosity have better osteogenic microenvironment, so that the cells on the higher microporosity ceramics have stronger osteogenic differentiation ability, which has been proved in the previous research of our research group, and is consistent with the results of this study. ERK1/2 has been proved to be a classic protein that induces osteogenesis under mechanical stimulation, and it can also be activated as a downstream signal through the activation of piezo 1 or the influx of intracellular calcium. Mechanical stretching and hydrostatic pressure up-regulate bone-specific genes by activating ERK1/2, thus promoting osteogenesis. In addition, as a classic mechanosensitive receptor and upstream of ERK1/2 [54], FAK signal has also been observed to be involved in the regulation of cell proliferation, differentiation and apoptosis through mechanical stimulation [55]. It is reported that mechanical stretching induces osteogenesis of hBMSCs by enhancing the expression of H19 and downstream FAK proteins, graphene activates mechanically sensitive integrin-FAK axis to promote osteogenesis. However, according to the results of our work, the regulation of electrical signal waveform on the expression of ERK1/2 signal and FAK signal mainly affects the proliferation of cells, which is reflected in the continuous upregulation of experiential group.
The above results show that MVS-HA ceramic coupling driven by electrical signal waveform can exert a time sequential and fine regulatory effect on TRPV1/Piezo1/2-ERK1/2 and FAK-ERK1/2 signal axes. It gives endows cells with higher osteogenic activity or proliferative activity, and provides more evidence for a better understanding of the molecular mechanism of MV regulating cell and graft osteogenic behavior.
Finally, we evaluated the bone regeneration ability of bone grafts constructed by MVS driven by different waveform electrical signals coupled with ceramic porous scaffolds. The results of fluorescence in vivo showed that the square wave group always had the highest survival rate and proliferative activity, followed by the sine wave group. The triangle wave group had the weakest proliferative activity despite the high survival rate. This may be due to the fact that when triangle wave-driving MVS-HA materials are used to construct bone grafts in vitro, a large number of cells have started osteogenic differentiation prematurely, thus inhibiting cell proliferation. Some studies pointed out that the survival and proliferation of early implanted cells are essential for successful bone formation and implant integration, the defect site needs enough seed cells to initiate osteoblast differentiation and promote osteogenesis. The results of fluorescence semi-quantitative analysis showed that the proliferation ability of each experimental group in vivo was square wave group > sine wave group > triangle wave group, which was consistent with the results of in vitro experiment.
In particularly, the histological results show that the bone implants constructed by MV-Materials coupling driven by square wave have formed new bone tissue and mature blood vessels only 2 weeks after subcutaneous implantation in nude mice. The speed of osteogenesis is significantly better than that reported in the literature. Different degrees of osteogenesis were also observed in other experimental groups, which had never been reported before. Generally speaking, the osteogenic ability of ectopic osteogenesis in subcutaneous implantation is sparse compared with other ectopic osteogenic models due to the scarcity of mesenchymal cells and blood vessels [56]. The current time points for subcutaneous osteogenesis in nude mice are mostly at 4 W, 6 W and 8 W [[57], [58], [59]]. This may be because the implants in the square group have the highest proliferative activity while tolerating physiological and mechanical stimulation and hypoxia-ischemia, so that osteogenesis can be achieved earlier in the osteogenic microenvironment provided by MVS-HA ceramic scaffolds. Although the implant in the triangle wave group had earlier osteogenic ability, the inhibition of cell number and proliferation limited its osteogenic capacity. Therefore, although osteogenesis occurred 2 weeks after implantation, such osteogenic advantage was not be enhanced over time. The ability of cell proliferation and osteogenic differentiation of implants in sine wave group is in a balanced state. Therefore, although the formation of new bone is weaker than that in the triangle wave group at the early stage of implantation (2 weeks), with the extension of time, cells continue to proliferate, tissues grow into the internal area of the implant, and the scope of new bone formation also continues to expand.
The expression of bone specific protein showed a time sequence pattern, and with the passage of time, the high expression of BMP2, Col-I and OCN appeared in turn. The results showed that the pretreatment of implants by MVS did not change the characteristics of physiological development. The expression of specific bone protein of new bone formation is usually sequential, with high expression of Runx2 and BMP2 in the early stage, Col-I and ALP in the middle stage, and OPN, OCN in the late stage. OCN, in particular, is a landmark protein of osteoid maturation and mineralization rich in collagen. Interestingly, there was a significant high expression of OCN at two weeks after implantation in the square wave group, which corresponds to the results of histological staining, indicating that there was new bone that began to mineralize and mature in the bone graft as early as 2 weeks. This also explains why the expression of BMP2 in square wave group was lower than that in other wave groups at 2 weeks. The sustained high expression of Col-I and OCN in the square wave group indicates that the bone graft has a high speed of osteogenesis and a large amount of osteogenesis. Although the bone velocity of triangle wave group and sine wave group was weaker than square wave group, it was still significantly higher than the control group. The expression of BMP-2 in triangle wave group was the highest at 2 weeks, which was consistent with the experimental results in vitro, indicating that MVS driven by triangle wave coupled with materials promote osteogenic differentiation. However, it is possible that the trend of osteogenic differentiation suppresses the trend of proliferation, which results in less osteogenesis and less OCN secretion. Overall, our study shows that the osteogenic differentiation of BMSCs treated with MVS is consistent with the known pathway of osteogenic differentiation. In addition, angiogenesis plays an important role in bone formation, as previous studies have shown that angiogenic factors, such as vascular endothelial growth factor (VEGF) in implants, can effectively promote osteogenesis [40,60]. In this study, MVS bone grafts promote angiogenesis by increasing the number of capillaries and microvessels, and promoting the expression of NO, which is closely related to angiogenesis. The results in vitro showed iNOS gene was highly expressed in the coupling bone grafts, which indirectly indicated the high expression of NO. Studies have shown that NO can not only promote osteoblast bone differentiation, but also regulate angiogenesis in the local microenvironment [61].
In summary, as shown in Fig. 10, the HA ceramic-MVS coupling driven by the electrical signal waveform can regulate the calcium concentration and surface micro-morphology in the osteogenic microenvironment by affecting the calcium phosphorus dissolution and deposition of the HA ceramic scaffold, and regulate the FAK-ERK1/2 signal axis pathway and the cell calcium channel TRPV1/Piezo1/Piezo2-ERK1/2 sig-nal axis pathway through the changes of fluid shear stress and calcium ion concentra-tion to play a fine sequential intervention on osteoblasts, thus endowing bone grafts with differentiated and rapid osteogenic abilities. Future studies will further explore the effects of electrical signal waveform-driving MVS coupled with calcium phosphate ceramics on immune response and osteogenic balance, as well as the time sequential combination of coupling effects driven by electrical signal waveform, in order to achieve a better function of osteogenesis.
Fig. 10.
Different Electrical signal waveform adjust external force field to construct functional tissue engineered bone with rapid osteogenic properties by coupled calcium phosphate bio-ceramics.
5. Conclusion
Our findings demonstrated that MVS driven by different electrical signal waveforms have different intervention effects on the biochemical and biophysical characteristics of osteogenic microenvironment on HA ceramic to regulate the phosphorylation of mechanical transduction signal pathways and the activation of calcium channels on cell membrane, which further promote bone formation in a time-sequential way. This work provides a non-invasive and non-additive ideal research model for exploring the optimization strategy of osteogenic microenvironment under MVS and constructing implants for rapid bone regeneration.
CRediT authorship contribution statement
Yuehao Wu: Data curation, Investigation, Methodology, Writing - original draft. Jinjie Wu: Data curation, Investigation. Xu Huang: Data curation, Validation, Visualization. Xiupeng Zhu: Data curation. Wei Zhi: Conceptualization, Project administration, Writing - review & editing. Jianxin Wang: Supervision, Writing - review & editing. Dong Sun: Resources, Writing - review & editing. Xuening Chen: Validation. Xiangdong Zhu: Writing - review & editing. Xingdong Zhang: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Analytical and Testing Center of Southwest Jiaotong University for the SEM analysis.
This work was supported by the National Key Research and Development Program of China [Grant No.2016YFC1102000]; the National Natural Science Foundation of China Youth Program [Grant No.82202707]; the National Natural Science Foundation of China [Grant No.82272515]; the Chongqing Natural Science Foundation [Grant No. cstc2020jcyj-msxmX0351].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2023.100891.
Contributor Information
Wei Zhi, Email: zhiwei@home.swjtu.edu.cn.
Jianxin Wang, Email: jwang@swjtu.edu.cn.
Dong Sun, Email: sumersun07@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Sodhi H., Panitch A. Glycosaminoglycans in tissue engineering: a review [J] Biomolecules. 2020;11(1) doi: 10.3390/biom11010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu T., Jiang M., Zhang M., et al. Biofunctionalized composite scaffold to potentiate osteoconductive, angiogenesis, and favorable metabolic microenvironment for osteonecrosis therapy [J] Bioact. Mater. 2022;9:446–460. doi: 10.1016/j.bioactmat.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang G., Li F., Zhao X., et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment [J] Chem. Rev. 2017;117(20):12764–12850. doi: 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel D.K., Dutta S.D., Hexiu J., et al. 3D-printable chitosan/silk fibroin/cellulose nanoparticle scaffolds for bone regeneration via M2 macrophage polarization [J] Carbohydr. Polym. 2022;281 doi: 10.1016/j.carbpol.2021.119077. [DOI] [PubMed] [Google Scholar]
- 5.Eivazzadeh-Keihan R., Maleki A., De La Guardia M., et al. Carbon based nanomaterials for tissue engineering of bone: building new bone on small black scaffolds: a review [J] J. Adv. Res. 2019;18:185–201. doi: 10.1016/j.jare.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diomede F., Marconi G.D., Fonticoli L., et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration [J] Int. J. Mol. Sci. 2020;21(9) doi: 10.3390/ijms21093242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heng B.C., Bai Y., Li X., et al. Animal Model Exp Med; 2023. The Bioelectrical Properties of Bone Tissue [J] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zan J., Qian G., Deng F., et al. Dilemma and breakthrough of biodegradable poly-l-lactic acid in bone tissue repair [J] J. Mater. Res. Technol. 2022;17:2369–2387. [Google Scholar]
- 9.Lisowska B., Kosson D., Domaracka K. Lights and shadows of NSAIDs in bone healing: the role of prostaglandins in bone metabolism [J] Drug Des. Dev. Ther. 2018;12:1753–1758. doi: 10.2147/DDDT.S164562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G.B., Xu R., Zhang C., et al. Responses of MSCs to 3D scaffold matrix mechanical properties under oscillatory perfusion culture [J] ACS Appl. Mater. Interfaces. 2017;9(2):1207–1218. doi: 10.1021/acsami.6b10745. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C.C., Wang X.Y., Gao L., et al. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells [J] Acta Biomater. 2018;73:509. doi: 10.1016/j.actbio.2018.04.030. -+ [DOI] [PubMed] [Google Scholar]
- 12.Kim M., Carman C.V., Springer T.A. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins [J] Science. 2003;301(5640):1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 13.Manske S.L., Good C.A., Zernicke R.F., et al. High-frequency, low-magnitude vibration does not prevent bone loss resulting from muscle disuse in mice following botulinum toxin injection [J] PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin A., Zhang P., Yang Y., et al. Research progress on mechanisms of vibration in osteogenesis of bone mesenchymal stem cells [J] J. Med. Biomechanics. 2019;34(4):440–445. [Google Scholar]
- 15.Lombardi M.L., Jaalouk D.E., Shanahan C.M., et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton [J] J. Biol. Chem. 2011;286(30):26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichenbach M., Mendez P.L., Madaleno C.D., et al. Differential impact of fluid shear stress and YAP/TAZ on BMP/TGF-β induced osteogenic target genes [J] Advanced Biology. 2021;5(2) doi: 10.1002/adbi.202000051. [DOI] [PubMed] [Google Scholar]
- 17.Choi R.B., Robling A.G. The Wnt pathway: an important control mechanism in bone's response to mechanical loading [J] Bone. 2021:153. doi: 10.1016/j.bone.2021.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P., Wu Y.Q., Jiang Z.L., et al. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling [J] Int. J. Mol. Med. 2012;29(6):1083–1089. doi: 10.3892/ijmm.2012.934. [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Chen T., Wang Z., et al. Joint construction of micro-vibration stimulation and BCP scaffolds for enhanced bioactivity and self-adaptability tissue engineered bone grafts [J] J. Mater. Chem. B. 2020;8(19):4278–4288. doi: 10.1039/d0tb00223b. [DOI] [PubMed] [Google Scholar]
- 20.Wu J., Tang Y., Pu X., et al. The role of micro-vibration parameters in inflammatory responses of macrophages cultured on biphasic calcium phosphate ceramics and the resultant influence on osteogenic differentiation of mesenchymal stem cells [J] J. Mater. Chem. B. 2021;9(38):8003–8013. doi: 10.1039/d1tb00898f. [DOI] [PubMed] [Google Scholar]
- 21.Wu J., Feng C., Wang M., et al. Whisker of biphasic calcium phosphate ceramics: osteo-immunomodulatory behaviors [J] Nano Res. 2022;15(10):9169–9182. [Google Scholar]
- 22.Zhang C.X., Lu Y.Q., Zhang L.K., et al. Influence of different intensities of vibration on proliferation and differentiation of human periodontal ligament stem cells [J] Arch. Med. Sci. 2015;11(3):638–646. doi: 10.5114/aoms.2015.52370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pre D., Ceccarelli G., Visai L., et al. High-frequency vibration treatment of human bone marrow stromal cells increases differentiation toward bone tissue [J] Bone marrow research. 2013;2013 doi: 10.1155/2013/803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orapiriyakul W., Tsimbouri M.P., Childs P., et al. Nanovibrational stimulation of mesenchymal stem cells induces therapeutic reactive oxygen species and inflammation for three-dimensional bone tissue engineering [J] ACS Nano. 2020;14(8):10027–10044. doi: 10.1021/acsnano.0c03130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H.X., Zhou K.X., Wang M.F., et al. Integrating physicomechanical and biological strategies for BTE: biomaterials-induced osteogenic differentiation of MSCs [J] THERANOSTICS. 2023;13(10):3245–3275. doi: 10.7150/thno.84759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabetti S., Sileo A., Montrone F., et al. Versatile electrical stimulator for cardiac tissue engineering-Investigation of charge-balanced monophasic and biphasic electrical stimulations [J] Front. Bioeng. Biotechnol. 2023:10. doi: 10.3389/fbioe.2022.1031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu G., Farahani F., Parra L.C. Cutaneous sensation of electrical stimulation waveforms [J] Brain Stimul. 2021;14(3):693–702. doi: 10.1016/j.brs.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M., Li J., Xiong X., et al. Heparinized PGA host-guest hydrogel loaded with paracrine products from electrically stimulated adipose-derived mesenchymal stem cells for enhanced wound repair [J] Engineered Regeneration. 2023;4(3):225–237. [Google Scholar]
- 29.Chen X., Ping Y. Wearable bioelectronic system for wound healing and management [J] Biomater Transl. 2023;4(2):65–66. doi: 10.12336/biomatertransl.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J., Wang J.Q., Ge B.F., et al. Different electromagnetic field waveforms have different effects on proliferation, differentiation and mineralization of osteoblasts in vitro [J] Bioelectromagnetics. 2014;35(1):30–38. doi: 10.1002/bem.21794. [DOI] [PubMed] [Google Scholar]
- 31.Xie C., Ye J.C., Liang R.J., et al. Advanced strategies of biomimetic tissue-engineered grafts for bone regeneration [J] Adcanced Healthcare Materials. 2021;10(14) doi: 10.1002/adhm.202100408. [DOI] [PubMed] [Google Scholar]
- 32.Steijvers E., Ghei A., Xia Z. Manufacturing artificial bone allografts: a perspective [J] Biomaterials translational. 2022;3(1):65–80. doi: 10.12336/biomatertransl.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul G.R., Malhotra A., Müller R. Mechanical stimuli in the local in vivo environment in bone: computational approaches linking organ-scale loads to cellular signals [J] Curr. Osteoporos. Rep. 2018;16(4):395–403. doi: 10.1007/s11914-018-0448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Lopez S., Villanueva R.E., Masso-Rojas F., et al. Micro-vibrations at 30Hz on bone cells cultivated in vitro produce soluble factors for osteoclast inhibition and osteoblast activity [J] Arch. Oral Biol. 2020;110 doi: 10.1016/j.archoralbio.2019.104594. [DOI] [PubMed] [Google Scholar]
- 35.Yang K., Li C.M., Wang Y.P., et al. Micro-vibration environment promotes bone marrow mesenchymal stem cells (BMSCs) healing of fracture ends and matrix metalloproteinase-9 (MMP-9) expression [J] JOURNAL OF BIOMATERIALS AND TISSUE ENGINEERING. 2022;12(6):1169–1174. [Google Scholar]
- 36.Chow S.K., Chim Y.N., Wang J., et al. Vibration treatment modulates macrophage polarisation and enhances early inflammatory response in oestrogen-deficient osteoporotic-fracture healing [J] Eur. Cell. Mater. 2019;38:228–245. doi: 10.22203/eCM.v038a16. [DOI] [PubMed] [Google Scholar]
- 37.Hua S.B., Su J., Deng Z.L., et al. Microstructures and properties of 45S5 bioglass (R) & BCP bioceramic scaffolds fabricated by digital light processing [J] Addit. Manuf. 2021:45. [Google Scholar]
- 38.Li H.Y., Chang J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic [J] Acta Biomater. 2013;9(2):5379–5389. doi: 10.1016/j.actbio.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Wu J., Zhang R., Li Y., et al. Reinforcing the function of bone graft via the Ca-P ceramics dynamic behavior-enhanced osteogenic microenvironment for optimal bone regeneration and reconstruction [J] Appl. Mater. Today. 2022;27 [Google Scholar]
- 40.Wu J., Wang M., Wu Y., et al. Coupling BCP ceramics with micro‐vibration stimulation field for cascade amplification from immune activation to bone regeneration [J] Adv. Funct. Mater. 2023 [Google Scholar]
- 41.Zhi W., Wang X., Sun D., et al. Optimal regenerative repair of large segmental bone defect in a goat model with osteoinductive calcium phosphate bioceramic implants [J] Bioact. Mater. 2022;11:240–253. doi: 10.1016/j.bioactmat.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang H., Xiang H.B., Yao Z.L., et al. Strontium-substituted calcium sulfate hemihydrate/hydroxyapatite scaffold enhances bone regeneration by recruiting bone mesenchymal stromal cells [J] J. Biomater. Appl. 2020;35(1):97–107. doi: 10.1177/0885328220915816. [DOI] [PubMed] [Google Scholar]
- 43.Li X., Wang Y., Chen F., et al. Design of macropore structure and micro-nano topography to promote the early neovascularization and osteoinductivity of biphasic calcium phosphate bioceramics [J] Mater. Des. 2022:216. [Google Scholar]
- 44.Xiong Y., Huang J., Fu L., et al. Enhancement of osteoblast cells osteogenic differentiation and bone regeneration by hydroxyapatite/phosphoester modified poly(amino acid) [J] Mater. Sci. Eng., C. 2020;111 doi: 10.1016/j.msec.2020.110769. [DOI] [PubMed] [Google Scholar]
- 45.Lee S.G., Lee S.N., Baek J., et al. Mechanical compression enhances ciliary beating through cytoskeleton remodeling in human nasal epithelial cells [J] Acta Biomater. 2021;128:346–356. doi: 10.1016/j.actbio.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Du R., Li D., Huang Y., et al. Effect of mechanical stretching and substrate stiffness on the morphology, cytoskeleton and nuclear shape of corneal endothelial cells [J] Medicine in Novel Technology and Devices. 2022;16 [Google Scholar]
- 47.Habibovic P., Yuan H., Van Der Valk C.M., et al. 3D microenvironment as essential element for osteoinductive by biomaterials [J] Biomaterials. 2005;26(17):3565–3575. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 48.Hou W., Zhang D., Feng X., et al. Low magnitude high frequency vibration promotes chondrogenic differentiation of bone marrow stem cells with involvement of beta-catenin signaling pathway [J] Arch. Oral Biol. 2020;118 doi: 10.1016/j.archoralbio.2020.104860. [DOI] [PubMed] [Google Scholar]
- 49.Lim J., Tai H.H., Liao W.H., et al. ASIC1a is required for neuronal activation via low-intensity ultrasound stimulation in mouse brain [J] Elife. 2021:10. doi: 10.7554/eLife.61660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao R., Yu B., Cui J., et al. Piezoelectric stimulation from electrospun composite nanofibers for rapid peripheral nerve regeneration [J] Nano Energy. 2022:98. [Google Scholar]
- 51.Liu Y., Tian H., Hu Y., et al. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing [J] Int. J. Biol. Sci. 2022;18(10):3961–3980. doi: 10.7150/ijbs.71390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolgorukova A., Isaeva J.E., Verbitskaya E., et al. Differential effects of the Piezo1 agonist Yoda1 in the trigeminovascular system: an electrophysiological and intravital microscopy study in rats [J] Exp. Neurol. 2021;339 doi: 10.1016/j.expneurol.2021.113634. [DOI] [PubMed] [Google Scholar]
- 53.Zhu D., Zhang G., Guo X., et al. A new hope in spinal degenerative diseases: piezo1 [J] BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6645193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun J., Luo Q., Liu L., et al. Low-level shear stress promotes migration of liver cancer stem cells via the FAK-ERK1/2 signalling pathway [J] Cancer Lett. 2018;427:1–8. doi: 10.1016/j.canlet.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Wang F., Wang Q., Zhao Y., et al. Adipose-derived stem cells with miR-150-5p inhibition laden in hydroxyapatite/tricalcium phosphate ceramic powders promote osteogenesis via regulating Notch3 and activating FAK/ERK and RhoA [J] Acta Biomater. 2022 doi: 10.1016/j.actbio.2022.09.070. [DOI] [PubMed] [Google Scholar]
- 56.Salazar V.S., Gamer L.W., Rosen V. BMP signalling in skeletal development, disease and repair [J] Nat. Rev. Endocrinol. 2016;12(4):203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 57.Hall G.N., Tam W.L., Andrikopoulos K.S., et al. Patterned, organoid-based cartilaginous implants exhibit zone specific functionality forming osteochondral-like tissues in vivo [J] Biomaterials. 2021;273 doi: 10.1016/j.biomaterials.2021.120820. [DOI] [PubMed] [Google Scholar]
- 58.Fang T., Yuan Z., Zhao Y., et al. Synergistic effect of stem cells from human exfoliated deciduous teeth and rhBMP-2 delivered by injectable nanofibrous microspheres with different surface modifications on vascularized bone regeneration [J] Chem. Eng. J. 2019;370:573–586. [Google Scholar]
- 59.Wang Y., Yuan S., Sun J., et al. Inhibitory effect of the TSG-6 on the BMP-4/Smad signaling pathway and odonto/osteogenic differentiation of dental pulp stem cells [J] Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110266. [DOI] [PubMed] [Google Scholar]
- 60.Zhuang Y., Liu A., Jiang S., et al. Promoting vascularized bone regeneration via strontium-incorporated hydroxyapatite bioceramic [J] Mater. Des. 2023:234. [Google Scholar]
- 61.Xu T.P., Yang Y.H., Suo D., et al. Electrosprayed regeneration-enhancer-element microspheres power osteogenesis and angiogenesis coupling [J] Small. 2022;18(36) doi: 10.1002/smll.202200314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.