Abstract
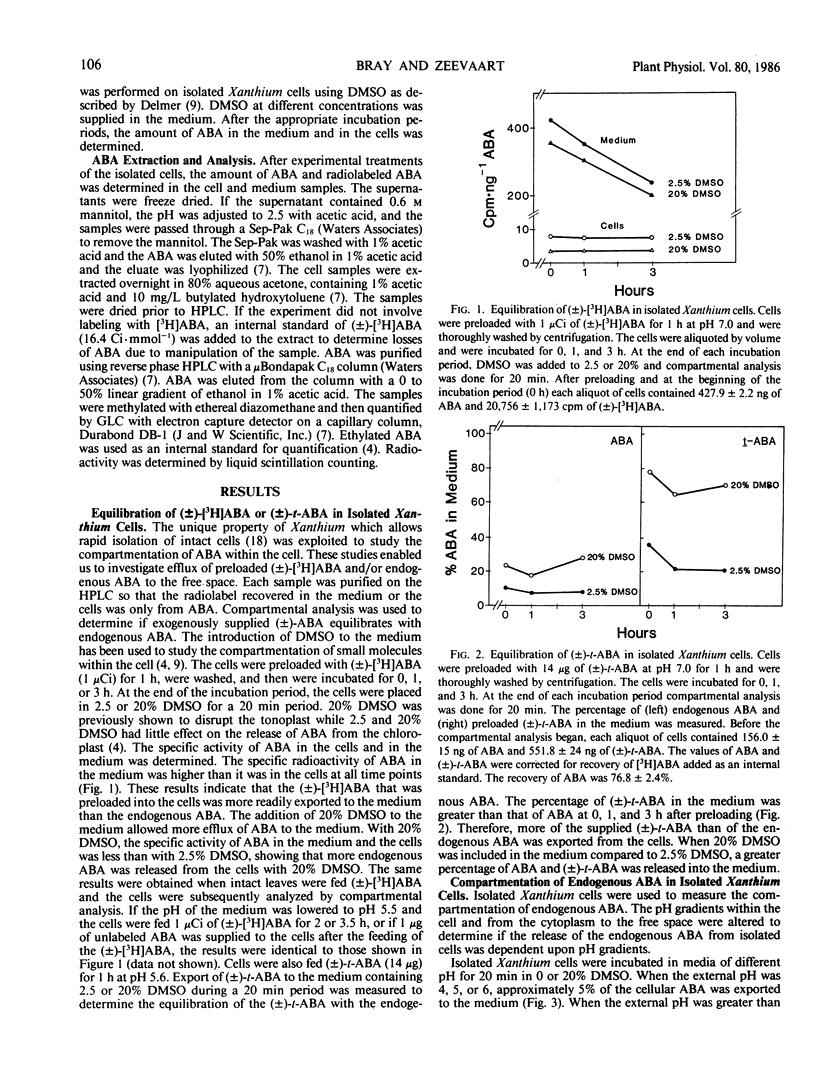
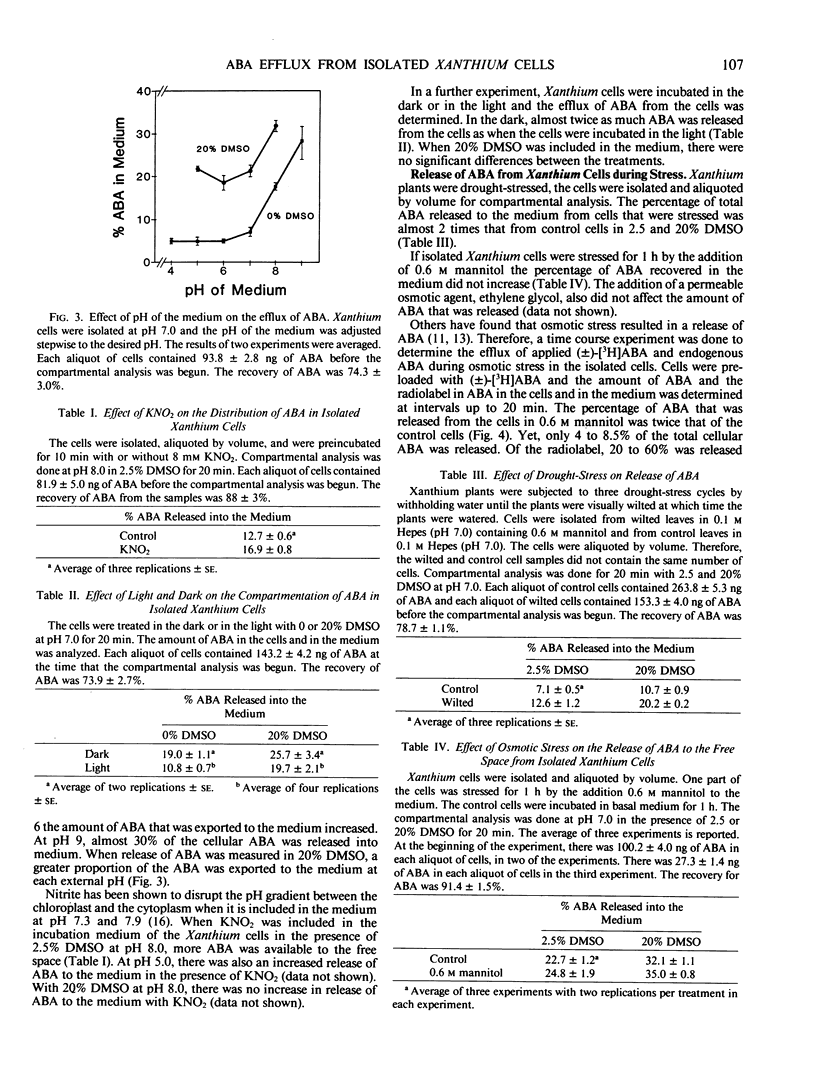
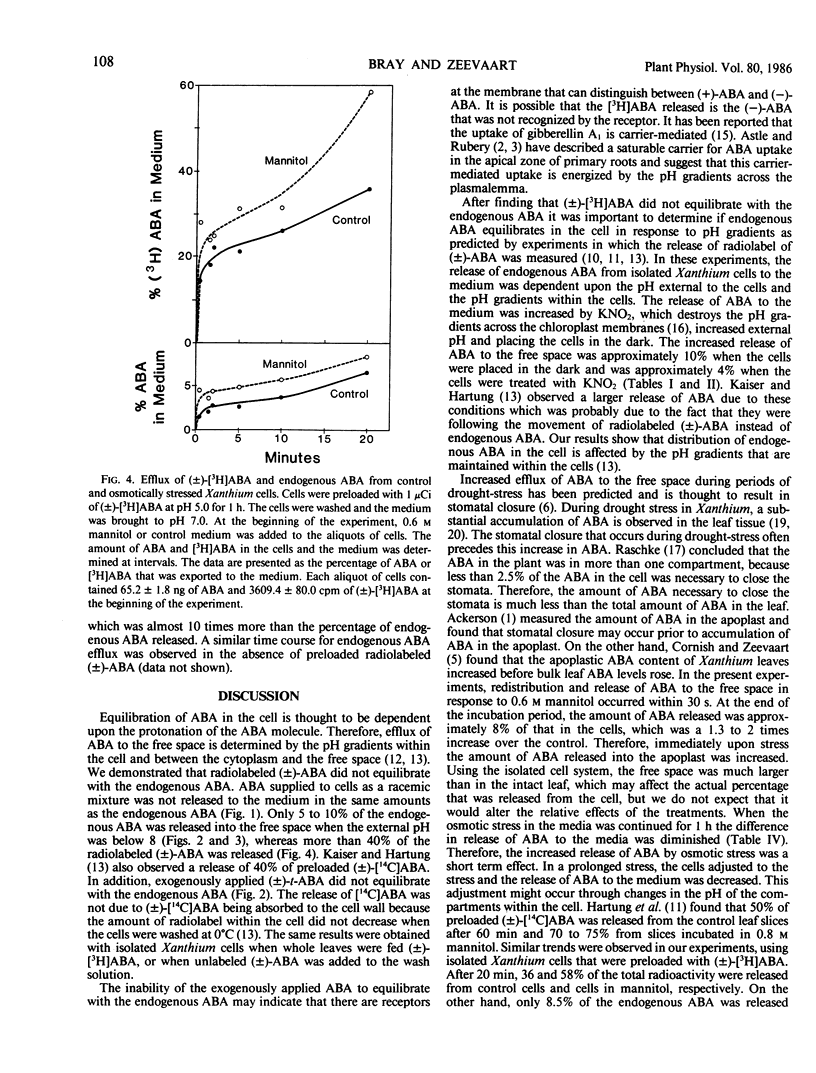
The compartmentation of endogenous abscisic acid (ABA), applied (±)-[3H]ABA, and (±)-trans-ABA was measured in isolated mesophyll cells of the Chicago strain of Xanthium strumarium L. The release of ABA to the medium in the presence or absence of DMSO was used to determine the equilibration of ABA in the cells. It was found that a greater percentage of the (±)-[3H]ABA and the (±)-trans-ABA was released into the medium than of the endogenous ABA, indicating that applied ABA did not equilibrate with the endogenous material.
Therefore, in further investigations only the compartmentation of endogenous ABA was studied. Endogenous ABA was released from Xanthium cells according to the pH gradients among the various cellular compartments. Thus, darkness, high external pH, KNO2, and droughtstress all increased the efflux of ABA from the cells. Efflux of ABA from the cells in the presence of 0.6 m mannitol occurred within 30 seconds, but only 8% of the endogenous material was released during the 20 minute treatment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerson R. C. Synthesis and movement of abscisic Acid in water-stressed cotton leaves. Plant Physiol. 1982 Mar;69(3):609–613. doi: 10.1104/pp.69.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E. A., Zeevaart J. A. The Compartmentation of Abscisic Acid and beta-d-Glucopyranosyl Abscisate in Mesophyll Cells. Plant Physiol. 1985 Nov;79(3):719–722. doi: 10.1104/pp.79.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Movement of Abscisic Acid into the Apoplast in Response to Water Stress in Xanthium strumarium L. Plant Physiol. 1985 Jul;78(3):623–626. doi: 10.1104/pp.78.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Abscisic Acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol. 1985 Jan;77(1):25–28. doi: 10.1104/pp.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. Dimethylsulfoxide as a potential tool for analysis of compartmentation in living plant cells. Plant Physiol. 1979 Oct;64(4):623–629. doi: 10.1104/pp.64.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Hartung W. Uptake and Release of Abscisic Acid by Isolated Photoautotrophic Mesophyll Cells, Depending on pH Gradients. Plant Physiol. 1981 Jul;68(1):202–206. doi: 10.1104/pp.68.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purczeld P., Chon C. J., Portis A. R., Jr, Heldt H. W., Heber U. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma. Studies with nitrite-mediated proton transfer across the envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):488–498. doi: 10.1016/0005-2728(78)90116-0. [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Raschke K. Effects of phaseic Acid and dihydrophaseic Acid on stomata and the photosynthetic apparatus. Plant Physiol. 1980 Feb;65(2):291–297. doi: 10.1104/pp.65.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium strumarium during and after Water Stress. Plant Physiol. 1980 Oct;66(4):672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Metabolism of Abscisic Acid and Its Regulation in Xanthium Leaves during and after Water Stress. Plant Physiol. 1983 Mar;71(3):477–481. doi: 10.1104/pp.71.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


