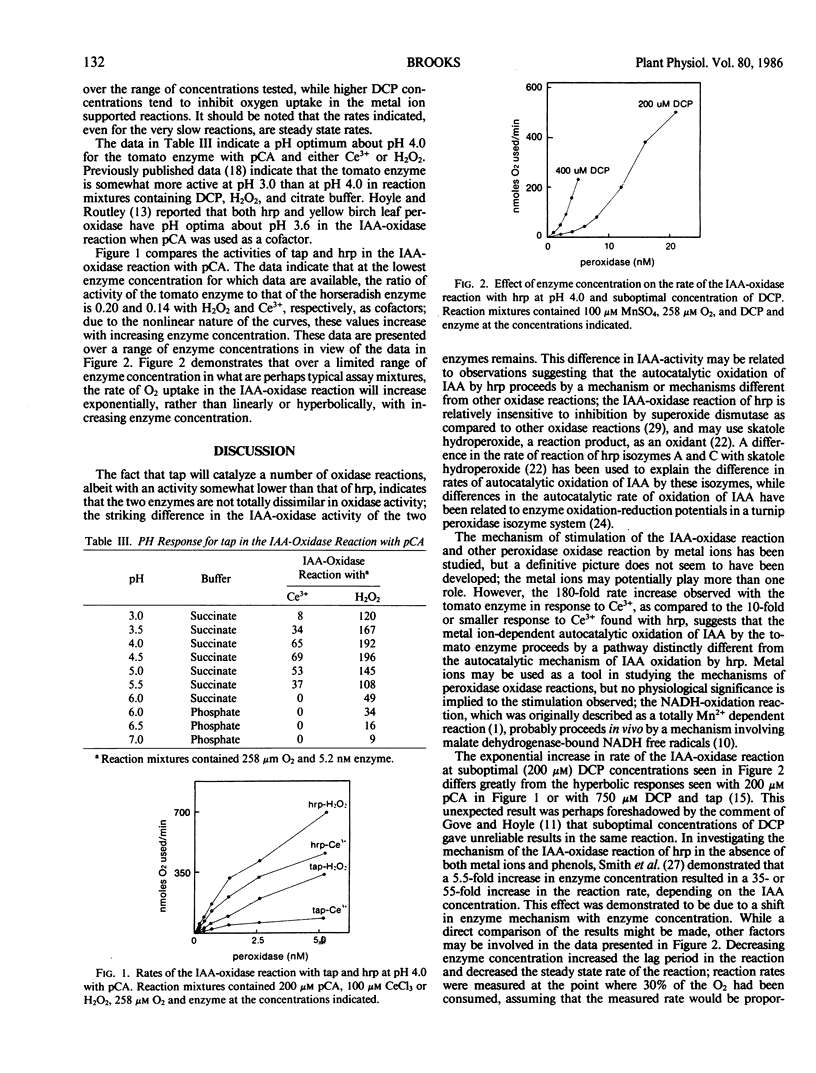
Abstract
Tomato (Lycopersicon esculentum Mill) anionic peroxidase was found to catalyze oxidase reactions with NADH, glutathione, dithiothreitol, oxaloacetate, and hydroquinone as substrates with a mean activity 30% that of horseradish peroxidase; this is in contrast to the negligible activity of the tomato enzyme as compared to the horseradish enzyme in catalyzing an indoleacetic acid-oxidase reaction with only Mn2+ and a phenol as cofactors. Substitution of Ce3+ for Mn2+ produced an 18-fold larger response with the tomato enzyme than with the horseradish enzyme, suggesting a significant difference in the autocatalytic indoleacetic acid-oxidase reactions with these two enzymes. In attempting to compare enzyme activities with 2,4-dichlorophenol as a cofactor, it was found that reaction rates increased exponentially with both increasing cofactor concentration and increasing enzyme concentration. While the former response may be analogous to allosteric control of enzyme activity, the latter response is contrary to the principle that reaction rate is proportional to enzyme concentration, and additionally makes any comparison of enzyme activity difficult.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAZAWA T., CONN E. E. The oxidation of reduced pyridine nucleotides by peroxidase. J Biol Chem. 1958 May;232(1):403–415. [PubMed] [Google Scholar]
- Boyer N., Chapelle B. Lithium Inhibition of the Thigmomorphogenetic Response in Bryonia dioica. Plant Physiol. 1979 Jun;63(6):1215–1216. doi: 10.1104/pp.63.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977 Mar;59(3):411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. L. Stimulation of peroxidase reactions by hydroxamates. Biochem Biophys Res Commun. 1983 Nov 15;116(3):916–921. doi: 10.1016/s0006-291x(83)80229-0. [DOI] [PubMed] [Google Scholar]
- Chibbar R. N., van Huystee R. B. Characterization of peroxidase in plant cells. Plant Physiol. 1984 Aug;75(4):956–958. doi: 10.1104/pp.75.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. J. Spectral similarities and kinetic differences of two tomato plant peroxidase isoenzymes. Plant Physiol. 1970 Jan;45(1):66–69. doi: 10.1104/pp.45.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C., Haard N. F. Initiation of Ripening in Bartlett Pear with an Antiauxin alpha(p-Chlorophenoxy)isobutyric Acid. Plant Physiol. 1973 Oct;52(4):380–384. doi: 10.1104/pp.52.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C. Oxidative turnover of auxins in relation to the onset of ripening in bartlett pear. Plant Physiol. 1975 Mar;55(3):480–484. doi: 10.1104/pp.55.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gove J. P., Hoyle M. C. The isozymic similarity of indoleacetic Acid oxidase to peroxidase in birch and horseradish. Plant Physiol. 1975 Nov;56(5):684–687. doi: 10.1104/pp.56.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman R. T. Effects of Growth-regulating Substances on the Carbon Dioxide Evolution and Post-harvest Ripening of Tomatoes. Plant Physiol. 1959 Jan;34(1):65–72. doi: 10.1104/pp.34.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAPPER M. H., HACKETT D. P. THE OXIDATIC ACTIVITY OF HORSERADISH PEROXIDASE. I. OXIDATION OF HYDRO- AND NAPHTHOHYDROQUINONES. J Biol Chem. 1963 Nov;238:3736–3742. [PubMed] [Google Scholar]
- Kay E., Shannon L. M., Lew J. Y. Peroxidase isozymes from horseradish roots. II. Catalytic properties. J Biol Chem. 1967 May 25;242(10):2470–2473. [PubMed] [Google Scholar]
- Kokkinakis D. M., Brooks J. L. Hydrogen Peroxide-mediated Oxidation of Indole-3-acetic Acid by Tomato Peroxidase and Molecular Oxygen. Plant Physiol. 1979 Aug;64(2):220–223. doi: 10.1104/pp.64.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinakis D. M., Brooks J. L. Tomato peroxidase: purification, characterization, and catalytic properties. Plant Physiol. 1979 Jan;63(1):93–99. doi: 10.1104/pp.63.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD J. B., BURRIS R. H. Participation of metals in peroxidasecatalyzed oxidations. J Biol Chem. 1959 Oct;234:2774–2777. [PubMed] [Google Scholar]
- Macnicol P. K. Peroxidases of the Alaska pea (Pisum sativum L.). Enzymic properties and distribution within the plant. Arch Biochem Biophys. 1966 Nov;117(2):347–356. doi: 10.1016/0003-9861(66)90422-x. [DOI] [PubMed] [Google Scholar]
- Nakajima R., Yamazaki I. The mechanism of indole-3-acetic acid oxidation by horseradish peroxidases. J Biol Chem. 1979 Feb 10;254(3):872–878. [PubMed] [Google Scholar]
- Olsen J., Davis L. The oxidation of dithiothreitol by peroxidases and oxygen. Biochim Biophys Acta. 1976 Sep 14;445(2):324–329. doi: 10.1016/0005-2744(76)90086-3. [DOI] [PubMed] [Google Scholar]
- Ricard J., Mazza G., Williams R. J. Oxidation-reduction potentials and ionization states of two turnip peroxidases. Eur J Biochem. 1972 Aug 4;28(4):566–578. doi: 10.1111/j.1432-1033.1972.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Shigeoka S., Nakano Y., Kitaoka S. Purification and some properties of L-ascorbic-acid-specific peroxidase in Euglena gracilis Z. Arch Biochem Biophys. 1980 Apr 15;201(1):121–127. doi: 10.1016/0003-9861(80)90495-6. [DOI] [PubMed] [Google Scholar]
- Smith A. M., Morrison W. L., Milham P. J. Oxidation of indole-3-acetic acid by peroxidase: involvement of reduced peroxidase and compound III with superoxide as a product. Biochemistry. 1982 Aug 31;21(18):4414–4419. doi: 10.1021/bi00261a034. [DOI] [PubMed] [Google Scholar]
- Stonier T., Yang H. M. Studies on Auxin Protectors: XI. Inhibition of Peroxidase-Catalyzed Oxidation of Glutathione by Auxin Protectors and o-Dihydroxyphenols. Plant Physiol. 1973 Feb;51(2):391–395. doi: 10.1104/pp.51.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Yamazaki I. The reaction between indole 3-acetic acid and horseradish peroxidase. Arch Biochem Biophys. 1973 Jan;154(1):147–159. doi: 10.1016/0003-9861(73)90043-x. [DOI] [PubMed] [Google Scholar]


