Abstract
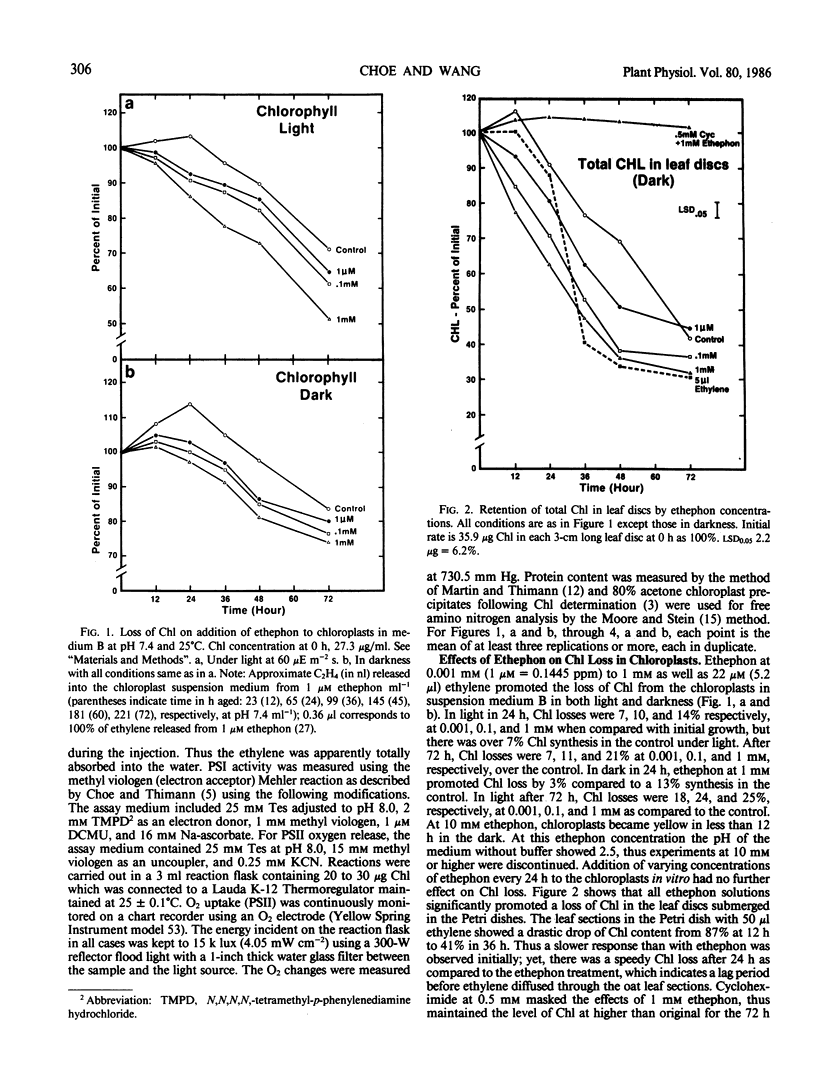
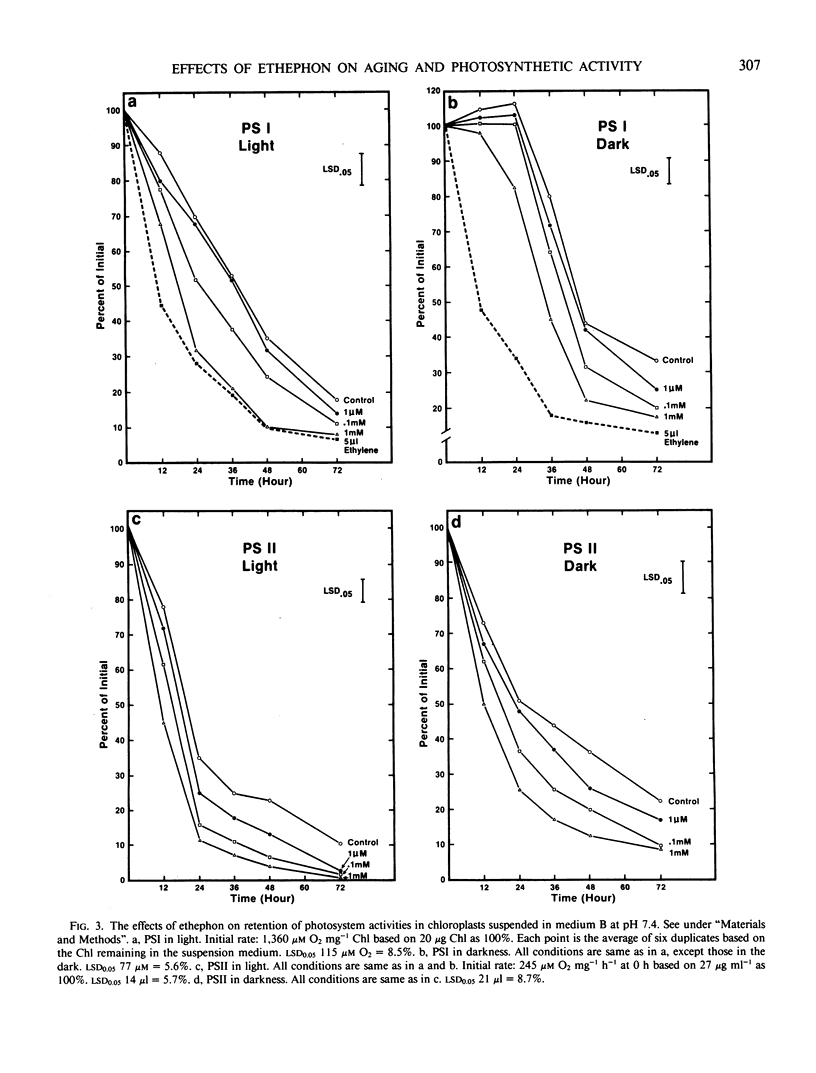
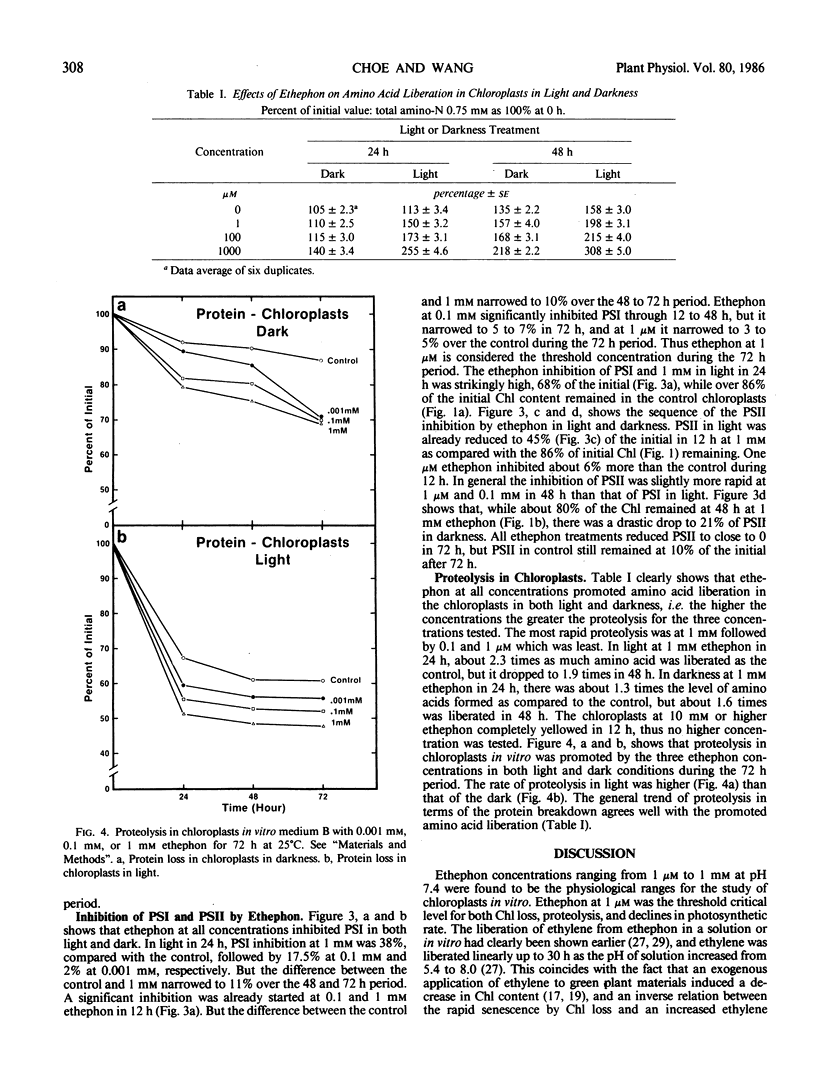
Chloroplasts, isolated from the primary leaves of 7-day-old seedlings, were incubated in vitro at 25°C with 2-chloroethylphosphonic acid (ethephon) under light (0.16 milliwatts per square centimeter) and dark conditions. Ethephon at 1 micromolar (0.1445 ppm), 0.1 and 1 millimolar, or 5 microliters ethylene promoted the deterioration of chloroplasts, increased proteolysis, and reduced the chlorophyll content and PSI and PSII during 72 hours under both light and dark conditions. The decline in PSI and PSII occurred prior to a measurable loss of chlorophyll. The loss of photosynthetic activity affected by ethephon was initiated prior to 12 hours of incubation. After 24 hours in light, 0.1 millimolar (1.445 ppm) epthephon significantly reduced PSI and PSII and promoted the total free amino acid liberation in isolated chloroplasts. In darkness the rate of loss of PSI activity was about 50% of that in light. After 24 hours, in light at 1 millimolar epthephon, PSII activity was 55% of the control, yet nearly 90% of the chlorophyll remained, which indicates that the loss of thylakoid integrity was promoted by ethephon. Ethylene injected in the chloroplast medium at 5 microliters (0.22 micromolar per milliliter) reduced PSI by nearly 50% of the initial in 12 hours. In leaf sections floated in 5 microliters per milliliter suspension medium, a 36% loss of chlorophyll of the control in 36 hours was observed. Cycloheximide at 0.5 millimolar masked the effect of 1 millimolar ethephon and maintained the initial chlorophyll content during the 72 hour period.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H. T., Thimann K. V. The Metabolism of Oat Leaves during Senescence: III. The Senescence of Isolated Chloroplasts. Plant Physiol. 1975 May;55(5):828–834. doi: 10.1104/pp.55.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S., Thimann K. V. The role of ethylene in the senescence of oat leaves. Plant Physiol. 1981 Aug;68(2):349–354. doi: 10.1104/pp.68.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giridhar G., Thimann K. V. Interaction between Senescence and Wounding in Oat Leaves. Plant Physiol. 1985 May;78(1):29–33. doi: 10.1104/pp.78.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Light-Stimulated Changes in the Acidity of Suspensions of Oat Protoplasts: Dependence upon Photosynthesis. Plant Physiol. 1983 Jun;72(2):351–355. doi: 10.1104/pp.72.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Lieberman M. Localization of the Ethylene-synthesizing System in Apple Tissue. Plant Physiol. 1977 Nov;60(5):794–799. doi: 10.1104/pp.60.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlasson W. B., Poovaiah B. W., Dostal H. C. Ethylene production and respiration in aging leaf segments and in disks of fruit tissue of normal and mutant tomatoes. Plant Physiol. 1975 Oct;56(4):547–549. doi: 10.1104/pp.56.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E., Kays S. J. Inhibition of photosynthesis by ethylene-a stomatal effect. Plant Physiol. 1982 Aug;70(2):598–601. doi: 10.1104/pp.70.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. C., Barmore C. R. Involvement of ethylene in chlorophyll degradation in peel of citrus fruits. Plant Physiol. 1981 Oct;68(4):854–856. doi: 10.1104/pp.68.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Hydrogen peroxide synthesis in isolated spinach chloroplast lamellae : an analysis of the mehler reaction in the presence of NADP reduction and ATP formation. Plant Physiol. 1982 Nov;70(5):1249–1254. doi: 10.1104/pp.70.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle J. C., Kende H. Ethylene Action and Loss of Membrane Integrity during Petal Senescence in Tradescantia. Plant Physiol. 1980 Jun;65(6):1067–1072. doi: 10.1104/pp.65.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. L., Leopold A. C. Ethylene evolution from 2-chloroethylphosphonic Acid. Plant Physiol. 1969 Jan;44(1):156–158. doi: 10.1104/pp.44.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Yang S. F. Ethylene evolution from 2-chloroethylphosphonic Acid. Plant Physiol. 1969 Aug;44(8):1203–1204. doi: 10.1104/pp.44.8.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]


