Abstract
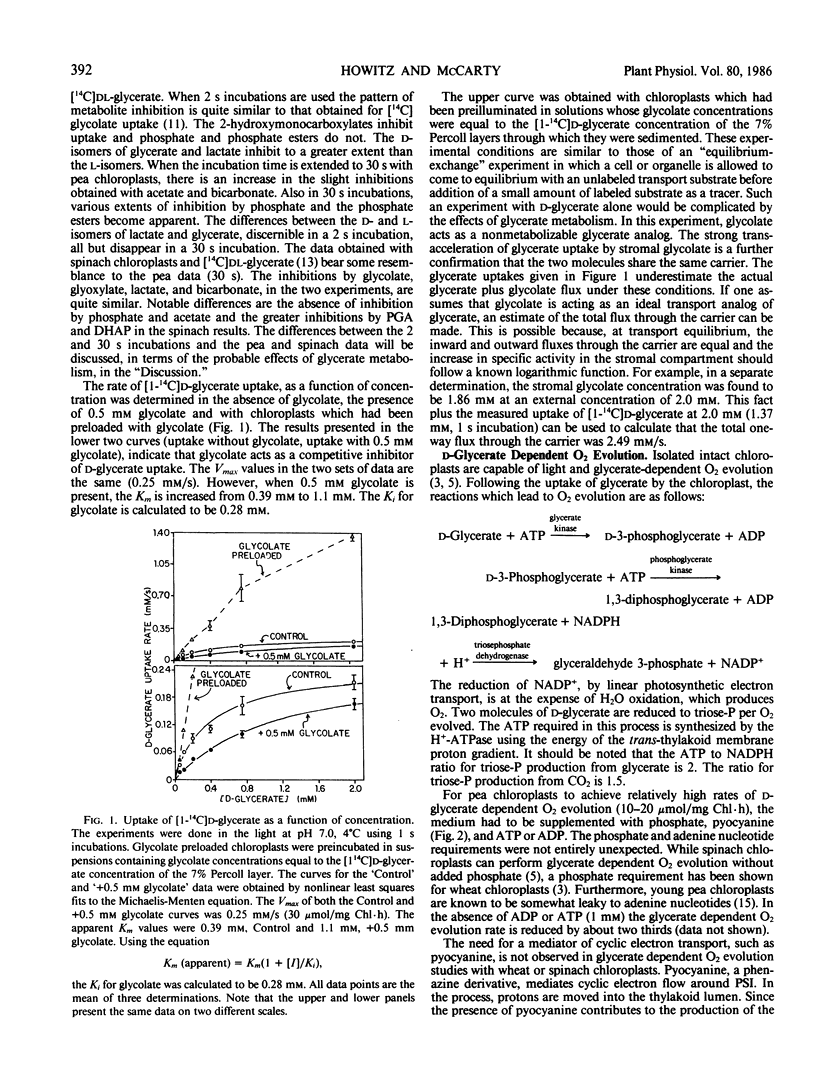
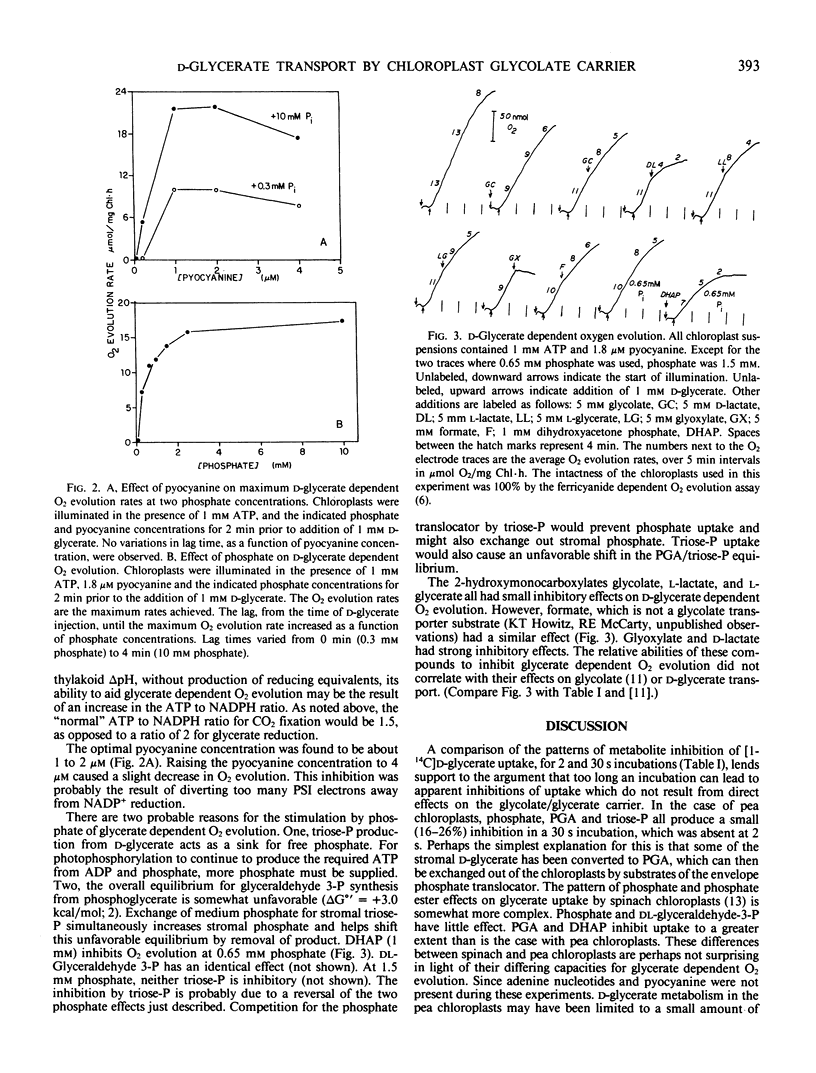
The transport of glycolate and d-glycerate, the substrate and end product of the photorespiratory carbon pathway, respectively, by isolated intact pea (Pisum sativum) chloroplasts has been compared. d-Glycerate uptake was inhibited by the 2-hydroxymonocarboxylates, glycolate, glyoxylate, and d-lactate. Phosphate and phosphoglycerate and triose phosphates were without effect when the assays were carried out for two seconds. Glycolate was found to be a competitive inhibitor of d-glycerate uptake and the presence of glycolate in the chloroplast stroma strongly enhanced d-glycerate uptake from the medium. For optimal rates of d-glycerate-dependent O2 evolution by pea chloroplasts, phosphate, ADP or ATP, and a mediator of cyclic electron flow had to be added. The inhibition of d-glycerate-dependent O2 evolution by triose phosphates and 2-hydroxymonocarboxylates was tested. The inhibition of d-glycerate-dependent O2 evolution by these metabolites did not correlate with their effects on glycerate transport. Thus, metabolism of d-glycerate, rather than its transport, limits the rate of glycerate-dependent oxygen evolution. The ramifications of d-glycerate metabolism on the interpretation of d-glycerate uptake data obtained with prolonged incubations are discussed. We conclude that d-glycerate and glycolate transport are mediated by the same transport system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards G. E., Walker D. A. Influence of glycerate on photosynthesis by wheat chloroplasts. Arch Biochem Biophys. 1984 May 15;231(1):124–135. doi: 10.1016/0003-9861(84)90369-2. [DOI] [PubMed] [Google Scholar]
- Enser U., Heber U. Metabolic regulation by pH gradients. Inhibition of photosynthesis by indirect proton transfer across the chloroplast envelope. Biochim Biophys Acta. 1980 Oct 3;592(3):577–591. doi: 10.1016/0005-2728(80)90102-4. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Howitz K. T., McCarty R. E. pH Dependence and Kinetics of Glycolate Uptake by Intact Pea Chloroplasts. Plant Physiol. 1982 Oct;70(4):949–952. doi: 10.1104/pp.70.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P. Lack of ATP requirement for light stimulation of glycerate transport into intact isolated chloroplasts. Plant Physiol. 1984 Jun;75(2):425–430. doi: 10.1104/pp.75.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P. Transport of Glycerate across the Envelope Membrane of Isolated Spinach Chloroplasts. Plant Physiol. 1982 Oct;70(4):1032–1038. doi: 10.1104/pp.70.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Stimulation of carbon dioxide fixation in isolated pea chloroplasts by catalytic amounts of adenine nucleotides. Plant Physiol. 1976 Aug;58(2):156–162. doi: 10.1104/pp.58.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]


