FIGURE 2.
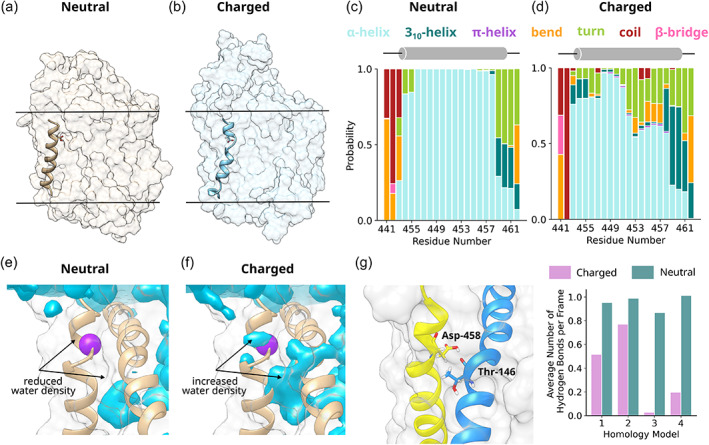
D458 protonation stabilizes transmembrane helix 9 (TM9). (a, b) Image showing the structure of TM9 in the presence of a neutral D458 (a) and a charged D458 (b). The hydrophobic boundary of the bilayer is indicated with a black line. (c, d) Bar graph showing the probability of residues 441–462 being in different secondary structural states for the neutral (c) and charged (d) D458, as defined by the Dictionary of Protein Secondary Structure (DSSP) algorithm (Kabsch & Sander, 1983). The data are averaged over four repeats. (e, f) Water density analysis showing water density in the presence of a (e) neutral aspartate and a (f) charged aspartate. In both cases, the data were averaged over four repeats. The location of D458 is indicated with a purple sphere. (h) A protonated aspartate side chain forms a hydrogen bond with the backbone carbonyl of T146, as shown in the left panel. The right panel shows the average number of hydrogen bonds per frame between D458 and T146 for the charged (pink) and neutral (teal) protonation states.
