FIGURE 5.
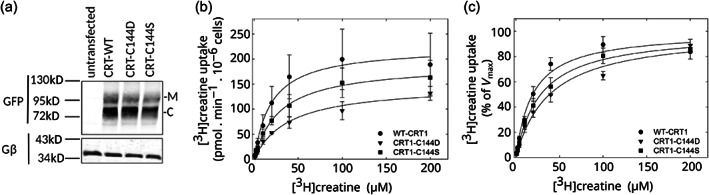
Experimental evidence against deprotonation of C144 in the substrate binding pocket of CRT1. (a) Immunoblotting for wild‐type CRT1 (WT), CRT1‐C144D (C144D), and CRT1‐C144S (C144S). Aliquots (10 μg) of lysates, which had been prepared from untransfected HEK293 cells and HEK293 cells transiently expressing YFP‐tagged wild type and mutant transporters, were processed as outlined in legend to Figure 2a. After transfer to nitrocellulose membranes, the immunoreactive bands were detected with an antibody directed against GFP (upper blot) or the G protein β‐subunits (as a loading control, lower blot). M and C indicate the positions of the (ER‐resident) core‐glycosylated species and the transporters harboring mature glycan moieties, respectively. (b) Saturation curves for substrate by wild‐type CRT1 (WT‐CRT1, circles), CRT1‐C144D (triangles), and CRT1‐C144S (squares). HEK293 cells were transfected with plasmids encoding YFP‐tagged wild type and mutant versions of CRT1. After 24 h, cells (2 × 105 /well) were seeded onto poly‐D‐lysine‐coated 48‐well plates and allowed to adhere overnight. Subsequently, the uptake reaction was initiated by adding [3H]creatine at the indicated concentration and carried out for 6 min as described under Section 5. Non‐specific uptake was determined in the presence of 300 μM β‐guanidinopropionic acid (<5% of total uptake) and was subtracted. Data are means ±SD from the three independent experiments carried out in triplicate. (c) Replot of the data shown in panel (b): uptake was normalized to the V max (=100%) calculated from fitting a hyperbola to the data points obtained in each individual uptake experiment. This normalization allowed for accounting for inter‐experimental variation in transfection efficiency and thus allowed for illustrating the rightward shift in K m resulting from the C144D mutation, which introduced a charge into the substrate binding pocket.
