Abstract
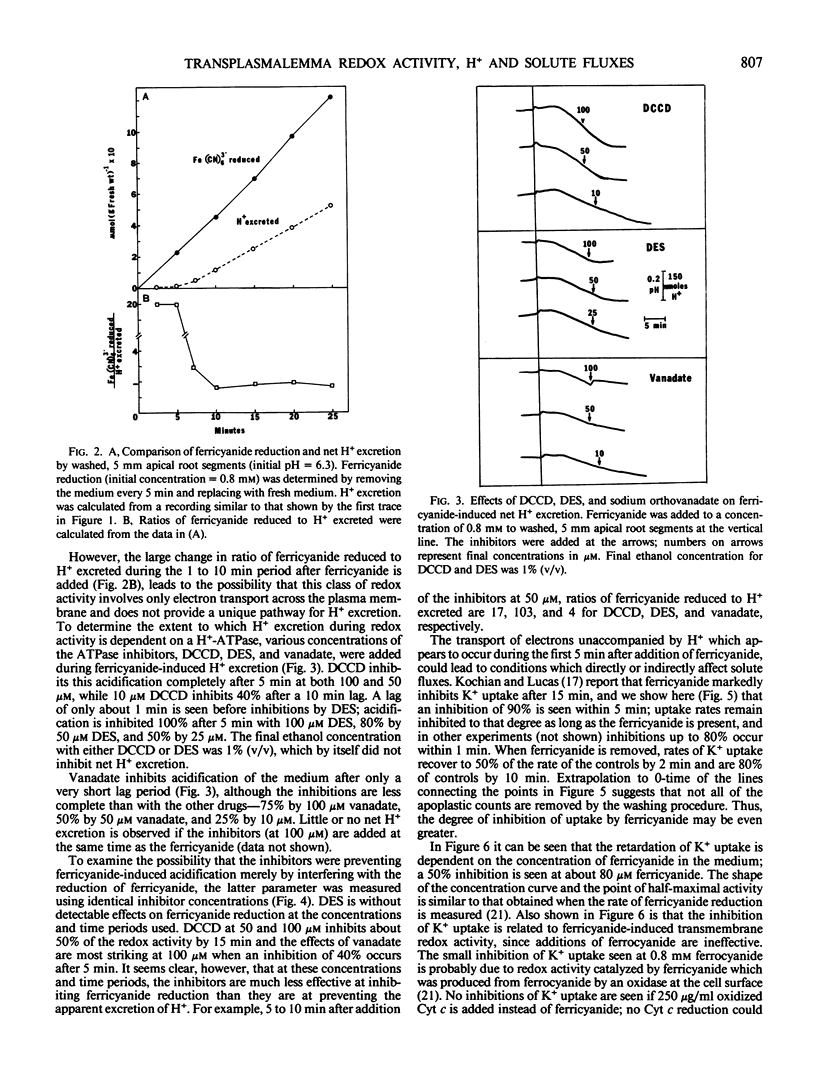
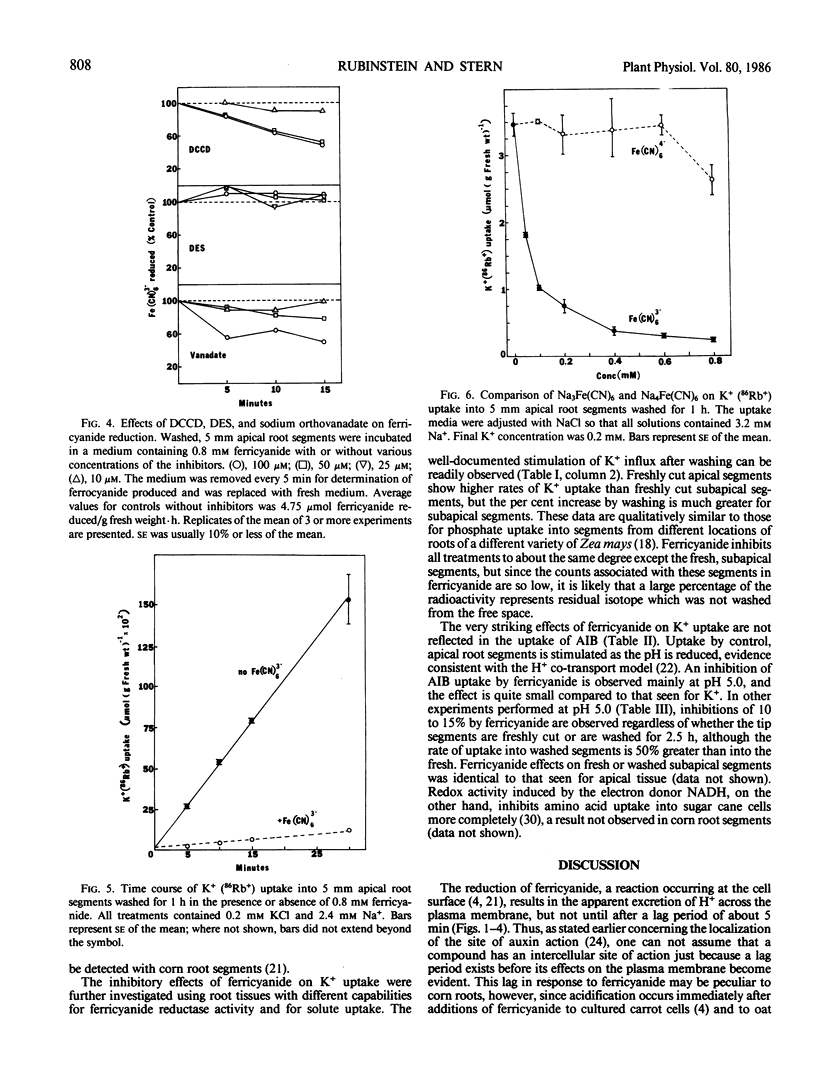
Transplasmalemma redox activity, monitored in the presence of exogenous ferricyanide stimulates net H+ excretion and inhibits the uptake of K+ and α-aminoisobutyric acid by freshly cut or washed, apical and subapical root segments of corn (Zea mays L. cv “Seneca Chief”). H+ excretion is seen only following a lag of about 5 minutes after ferricyanide addition, even though the reduction of ferricyanide occurs before 5 minutes and continues linearly. Once detected, the enhanced rate of H+ excretion is retarded by the ATPase inhibitors N,N′-dicyclohexylcarbodiimide, diethylstilbestrol, and vanadate. A model is presented in which plasmalemma redox activity in the presence of ferricyanide involves the transport only of electrons across the plasmalemma, resulting in a depolarization of the membrane potential and activation of an H+-ATPase. Such a model implies that this class of redox activity does not provide an additional and independent pathway for H+ transport, but that the activity may be an important regulator of H+ excretion. The 90% inhibition of K+ (86Rb+) uptake within 2 minutes after ferricyanide addition can be contrasted with the 5 to 15% inhibition of uptake of α-aminoisobutyric acid. The possibility exists that a portion of the K+ and most of the α-aminoisobutyric acid uptake inhibitions are related to the ferricyanide-induced depolarization of the membrane potential, but that the redox state of some component of the K+ uptake system may also regulate K+ fluxes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., SHAVIT N. A SENSITIVE AND SIMPLE METHOD FOR DETERMINATION OF FERROCYANIDE. Anal Biochem. 1963 Dec;6:549–554. doi: 10.1016/0003-2697(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Balke N. E., Hodges T. K. Effect of diethylstilbestrol on ion fluxes in oat roots. Plant Physiol. 1979 Jan;63(1):42–47. doi: 10.1104/pp.63.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R., Craig T. A., Crane F. L. Transmembrane ferricyanide reduction in carrot cells. Biochim Biophys Acta. 1985 Jan 10;812(1):49–54. doi: 10.1016/0005-2736(85)90520-6. [DOI] [PubMed] [Google Scholar]
- Bienfait H. F. Regulated redox processes at the plasmalemma of plant root cells and their function in iron uptake. J Bioenerg Biomembr. 1985 Apr;17(2):73–83. doi: 10.1007/BF00744199. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Cheeseman J. M., Lafayette P. R., Gronewald J. W., Hanson J. B. Effect of ATPase inhibitors on cell potential and k influx in corn roots. Plant Physiol. 1980 Jun;65(6):1139–1145. doi: 10.1104/pp.65.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane F. L., Sun I. L., Clark M. G., Grebing C., Löw H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta. 1985 Aug 1;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Federico R., Giartosio C. E. A transplasmamembrane electron transport system in maize roots. Plant Physiol. 1983 Sep;73(1):182–184. doi: 10.1104/pp.73.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Sebald W. The proton conducting F0-part of bacterial ATP synthases. Biochim Biophys Acta. 1984 Apr 9;768(1):1–27. doi: 10.1016/0304-4173(84)90005-3. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Taiz L. Vanadate inhibition of auxin-enhanced H secretion and elongation in pea epicotyls and oat coleoptiles. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7242–7246. doi: 10.1073/pnas.77.12.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium Transport in Corn Roots : III. Perturbation by Exogenous NADH and Ferricyanide. Plant Physiol. 1985 Feb;77(2):429–436. doi: 10.1104/pp.77.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium transport in corn roots : I. Resolution of kinetics into a saturable and linear component. Plant Physiol. 1982 Dec;70(6):1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Induction and development of increased ion absorption in corn root tissue. Plant Physiol. 1972 Mar;49(3):430–435. doi: 10.1104/pp.49.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Further Characterization on the Transport Property of Plasmalemma NADH Oxidation System in Isolated Corn Root Protoplasts. Plant Physiol. 1984 Feb;74(2):219–222. doi: 10.1104/pp.74.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Spanswick R. M. Effects of vanadate on the plasma membrane ATPase of red beet and corn. Plant Physiol. 1984 Jul;75(3):586–591. doi: 10.1104/pp.75.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Responses of Avena coleoptiles to suboptimal fusicoccin: kinetics and comparisons with indoleacetic Acid. Plant Physiol. 1981 Sep;68(3):543–547. doi: 10.1104/pp.68.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B., Stern A. I., Stout R. G. Redox activity at the surface of oat root cells. Plant Physiol. 1984 Oct;76(2):386–391. doi: 10.1104/pp.76.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Müller C., Marschner H. Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol. 1984 Nov;76(3):603–606. doi: 10.1104/pp.76.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs G., Spenney J. G., Lewin M. H+ transport: regulation and mechanism in gastric mucosa and membrane vesicles. Physiol Rev. 1978 Jan;58(1):106–173. doi: 10.1152/physrev.1978.58.1.106. [DOI] [PubMed] [Google Scholar]
- Sijmons P. C., Lanfermeijer F. C., de Boer A. H., Prins H. B., Bienfait H. F. Depolarization of Cell Membrane Potential during Trans-Plasma Membrane Electron Transfer to Extracellular Electron Acceptors in Iron-Deficient Roots of Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):943–946. doi: 10.1104/pp.76.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., van den Briel W., Bienfait H. F. Cytosolic NADPH is the electron donor for extracellular fe reduction in iron-deficient bean roots. Plant Physiol. 1984 May;75(1):219–221. doi: 10.1104/pp.75.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M., Maretzki A. Evidence for a plasmalemma redox system in sugarcane. Plant Physiol. 1985 Apr;77(4):873–876. doi: 10.1104/pp.77.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]


