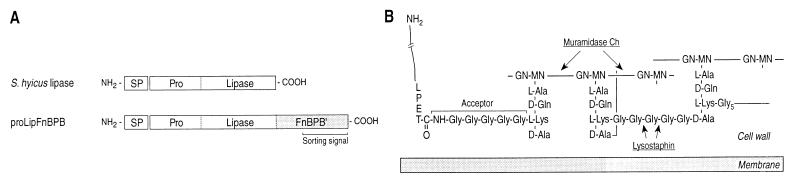
FIG. 1.
Structures of reporter proteins and the staphylococcal peptidoglycan. (A) Schematic diagrams showing the domains of the S. hyicus lipase and proLipFnBPB, a reporter enzyme for cell wall anchoring, consisting of the cleavable signal peptide (SP), the propeptide (Pro), and the catalytic domain (Lipase) of S. hyicus lipase fused to the C-terminal region of the S. aureus fibronectin binding protein B (FnBPB′). FnBPB′ comprises the complete cell wall-spanning region and the cell wall sorting signal of the binding protein. (B) Structure of staphylococcal peptidoglycan with a C-terminally processed surface protein attached to the lysostaphin-sensitive wild-type pentaglycine acceptor of a branched anchor peptide. Cleavage sites of cell wall lytic enzymes used in this study are indicated (modified after references 16 and 18).