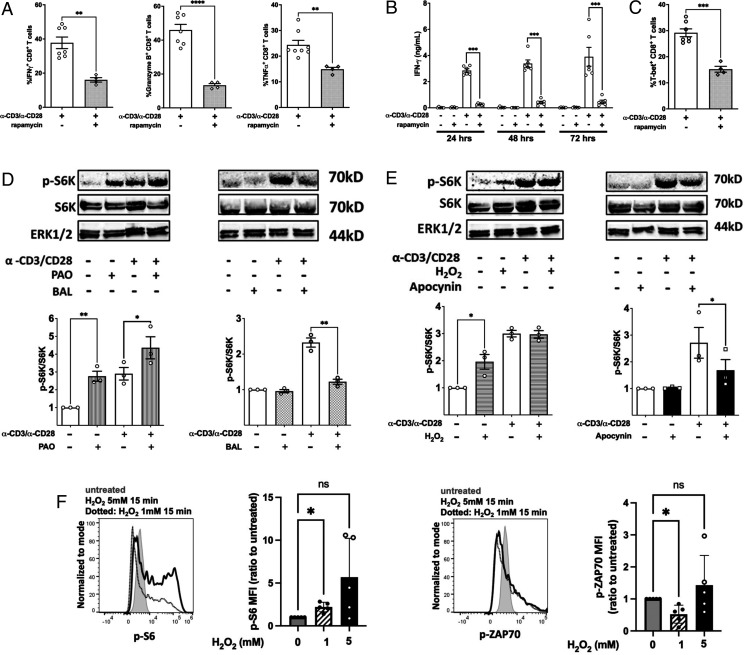
FIGURE 6.
mTORc is required for CD8+ T cell effector function through promoting transcriptional activity of T-bet through a redox-regulated mechanism. (A) Flow cytometry quantitation of intracellular staining of the IFN-γ, granzyme B, and TNF-α in purified NOD CD8+ T cells stimulated by α-CD3/α-CD28 for 66 h in the presence or absence of 20 ng/ml rapamycin followed by restimulation with PMA/ionomycin plus GolgiStop for 6 h. (B) NOD CD8+ T cells (5 × 104) were stimulated by α-CD3/α-CD28 in the presence or absence of 20 ng/ml rapamycin. IFN-γ was measured by ELISA on days 1, 2, and 3. (C) Intracellular staining for T-bet in CD8+ T cells after a 72-h polyclonal stimulation. NOD CD8+ T cells were treated with (D) PAO or BAL or (E) H2O2 or apocynin either with or without activation by α-CD3/α-CD28 for 30 min. Phosphorylation of S6K was measured by the ratio of phosphorylated S6K and total SK6. Results are from three independent experiments. Untreated NOD CD8+ T cells were used as the reference group for normalization among multiple tests. (F) H2O2 slightly increased p-S6 but not p-ZAP70 in human CD8+ T cells. Human PBMCs were treated with H2O2 at the indicated concentrations for 15 min at 37°C, and p-S6 and p-ZAP70 were stained with fluorescent Abs and detected with a flow cytometer. Shown are MFI ratio to untreated (first group). Gated on Live/Dead-Yellow−CD3+CD8+ T cells. Results are presented as mean ± SEM. Student t test was performed to exclude potential batch effects (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).