Abstract
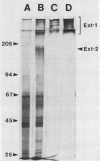
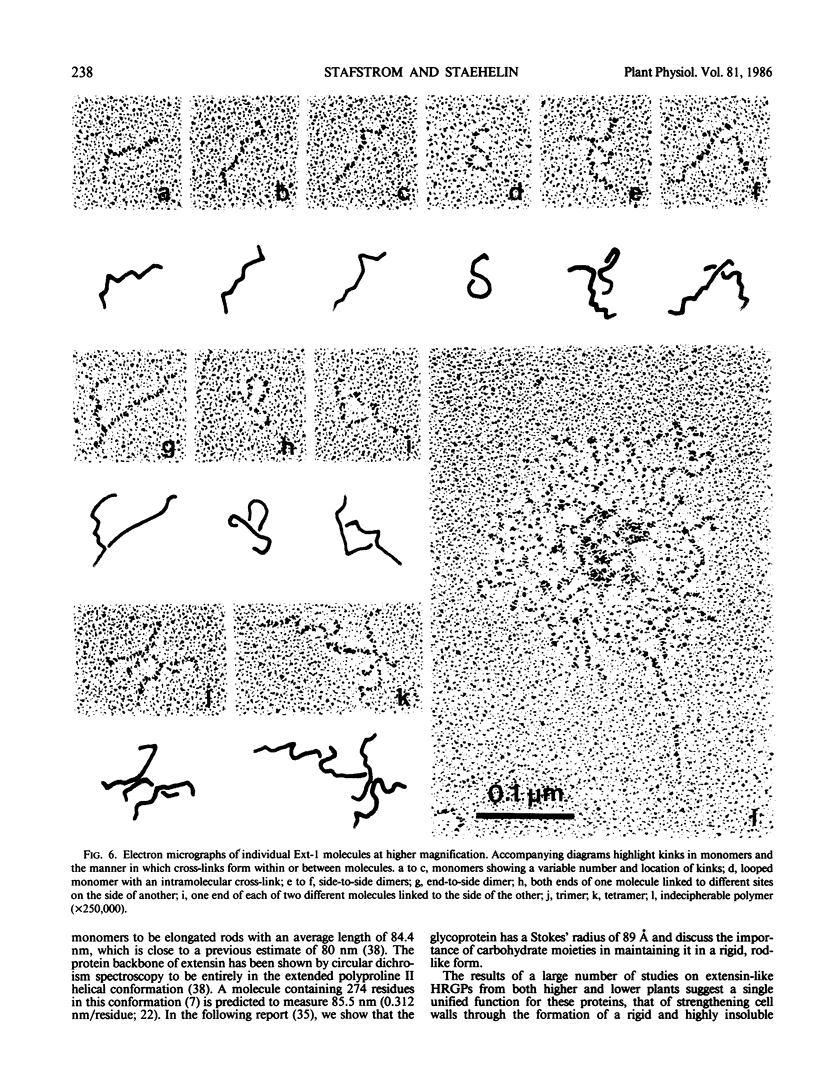
Extensins are hydroxyproline-rich glycoproteins (HRGPs) found in the primary cell walls of dicots. Extensin monomers are secreted into the wall and covalently bound to each other, presumably by isodityrosine (IDT) cross-links, to form a rigid matrix. Expression of the extensin matrix is correlated with inhibition of cell elongation during normal development and with increased resistance to virulent pathogens. We have isolated extensin from carrot root tissue (Daucus carota L.) by published techniques and have used gel filtration chromatography to purify fractions enriched in monomers and oligomers. We refer to this protein as “extensin-1” to distinguish it from “extensin-2,” a second extensin-like HRGP from carrot which we will describe later. We prepared extensin-1 for electron microscopy by shadowing it with platinum. Monomers are highly elongated (≅84 nanometers) and kinked at several sites. Kinks occur at all sites on molecules with nearly equal probability, but do not appear to occur at their ends. The distribution of kinks is similar to that of tyrosine-lysine-tyrosine sequences, which have been shown to be capable of forming intramolecular IDT cross-links, so we suggest that kinks are visible manifestations of intramolecular IDTs. Oligomers likely result from IDT cross-links between monomers, and may be regarded as transient precursors of the fully cross-linked matrix. Nearly 60% of cross-links involve the ends of molecules while the rest are scattered among internal sites. We discuss how the relative positions and proportions of intra- and intermolecular cross-links in extensin-1 may affect the structure, and in turn the function, of the extensin matrix.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysk M. M., Chrispeels M. J. Isolation and partial characterization of a hydroxyproline-rich cell wall glycoprotein and its cytoplasmic precursor. Biochim Biophys Acta. 1972 Feb 29;257(2):421–432. doi: 10.1016/0005-2795(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Cassab G. I., Nieto-Sotelo J., Cooper J. B., van Holst G. J., Varner J. E. A developmentally regulated hydroxyproline-rich glycoprotein from the cell walls of soybean seed coats. Plant Physiol. 1985 Mar;77(3):532–535. doi: 10.1104/pp.77.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Adair W. S., Mecham R. P., Heuser J. E. Chlamydomonas agglutinin is a hydroxyproline-rich glycoprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5898–5901. doi: 10.1073/pnas.80.19.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Cross-linking of soluble extensin in isolated cell walls. Plant Physiol. 1984 Oct;76(2):414–417. doi: 10.1104/pp.76.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Insolubilization of hydroxyproline-rich cell wall glycoprotein in aerated carrot root slices. Biochem Biophys Res Commun. 1983 Apr 15;112(1):161–167. doi: 10.1016/0006-291x(83)91811-9. [DOI] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamport D. T., Katona L., Roerig S. Galactosylserine in extensin. Biochem J. 1973 May;133(1):125–132. doi: 10.1042/bj1330125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Aman P., Franzén L. E., Albersheim P. Structural analysis of complex carbohydrates using high-performance liquid chromatography, gas chromatography, and mass spectrometry. Methods Enzymol. 1982;83:3–45. doi: 10.1016/0076-6879(82)83003-6. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- O'Neill M. A., Selvendran R. R. Glycoproteins from the cell wall of Phaseolus coccineus. Biochem J. 1980 Apr 1;187(1):53–63. doi: 10.1042/bj1870053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. Crystalline glycoprotein cell walls of algae: their stucture, composition and assembly. Philos Trans R Soc Lond B Biol Sci. 1974 Jul 25;268(891):129–146. doi: 10.1098/rstb.1974.0021. [DOI] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline-rich cell wall protein (extensin): role in the cessation of elongation in excised pea epicotyls. Dev Biol. 1973 Jan;30(1):49–55. doi: 10.1016/0012-1606(73)90047-x. [DOI] [PubMed] [Google Scholar]