Abstract
Objective
To compare the efficacy of laparoscopic pancreaticoduodenectomy (LPD) and open pancreaticoduodenectomy (OPD) in a medium-volume medical center.
Methods
Data for patients who underwent OPD or LPD for carcinoma of the ampulla of Vater (VPC) between January 2017 and June 2022 were acquired retrospectively. Propensity score-matching (PSM) analysis was performed to balance the baseline characteristics between the groups. The primary outcome was disease-free survival (DFS). Cox regression analysis was used to explore the independent risk factors for DFS.
Results
A total of 124 patients with pathologically diagnosed VPC were included. After 1:1 matching, there were 23 cases each in the OPD and LPD groups. Kaplan–Meier survival analyses showed that the median DFS in the OPD and LPD groups was identical (16.0 months vs 16.0 months, respectively). Multivariate Cox regression analysis showed that low levels of alkaline phosphatase and γ-glutamyl transpeptidase, positive surgical margin, and lymph node enlargement were independent risk factors for DFS.
Conclusion
LPD in medium-volume centers with acceptable technical conditions may approach or even achieve the efficacy of LPD in large-volume centers.
Keywords: Laparoscopy, pancreaticoduodenectomy, carcinoma of the ampulla of Vater, disease-free survival, complications, propensity score matching
Introduction
Carcinoma of the ampulla of Vater (VPC) refers to tumors that originate from one of the four types of epithelium in the region: the mucosa of the ampulla of Vater, pancreatic duct, distal common bile duct, or the periampullary duodenum. 1 Pancreaticoduodenectomy (PD) is the standard surgical approach for treating VPC. 2 With developments in laparoscopic surgery, laparoscopic pancreaticoduodenectomy (LPD) is currently an intensive area of research in hepatobiliary and pancreatic surgery.3–5 It is still controversial whether LPD has obvious advantages over open pancreaticoduodenectomy (OPD) regarding the incidence of postoperative complications and survival. 6 Most studies have shown that the rates of major complications and short-term survival are similar after the two types of surgery. 7 However, the study populations in recent studies were mainly concentrated in large-volume centers,8,9 and confounding factors and selection bias between groups were not well-controlled. Few such studies have been performed in medium-volume centers with acceptable technical conditions. Therefore, this study aimed to devise a novel method to analyze the efficacy of the two surgical methods for patients diagnosed with VPC. The study also aimed to explore the related factors affecting the prognosis in a medium-volume center to provide assistance in the treatment of this disease in medium-volume centers.
Methods
Patient selection and surgical procedure
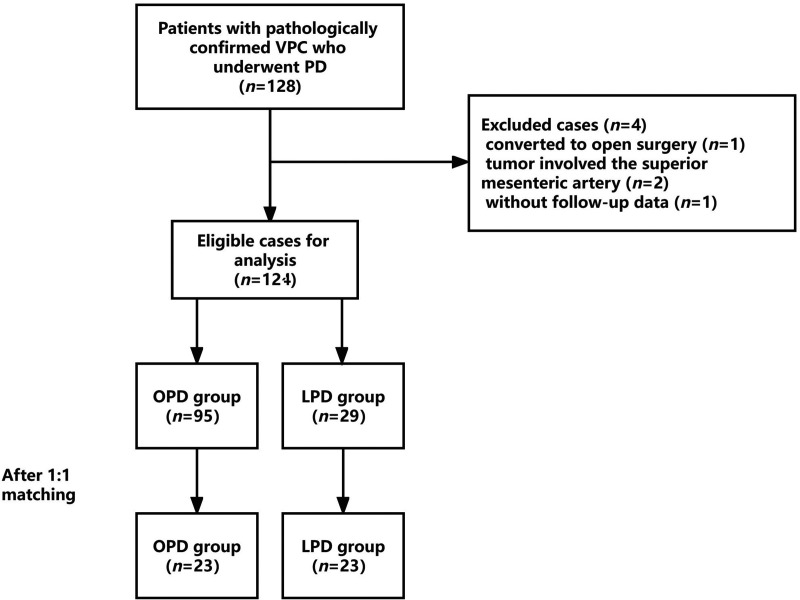
Data for patients who underwent PD in Shaoxing People’s Hospital from January 2017 to June 2022 were retrospectively collected. The patients were then divided into an LPD group (n = 29) and an OPD group (n = 95). A flowchart of the patient selection process is shown in Figure 1. The resectability status of the lesions was evaluated in accordance with the National Comprehensive Cancer Network criteria. 10 Patients in good general condition and with a confirmed diagnosis of VPC by postoperative pathology were included. Patients with pathologically confirmed benign or borderline diseases were excluded. Patients were also excluded if the tumor involved the celiac trunk and/or superior mesenteric artery.
Figure 1.
Flowchart of the patient selection process. VPC, carcinoma of the ampulla of Vater; PD, pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy; LPD, laparoscopic pancreaticoduodenectomy.
The reporting of this study conformed to the STROBE guidelines. 11 Our study was performed in accordance with the Helsinki Declaration of 1975 as revised in 2013. This study was approved by the Ethics Committee of Shaoxing People’s Hospital. Verbal consent was obtained from each patient, and we have de-identified all patient details. The technique we used for LPD was in accordance with the latest expert consensus on LPD in our country. 12 The OPD procedure resembled the LPD procedure.
Data collection
Clinicopathological data were acquired from our medical database. Preoperative baseline characteristics comprised age, sex, body mass index, preoperative biliary drainage, and laboratory data, such as carbohydrate antigen 199 (CA199), alkaline phosphatase (ALP), and γ-glutamyl transpeptidase (γ-GGT) levels, and the direct bilirubin-to-total bilirubin ratio (DBIL/TBIL). Intraoperative data comprised surgical margin status, tumor diameter, lymph node status, the tumor’s relationship with the superior mesenteric vein, operative time, and estimated blood loss volume. Enlarged lymph nodes were defined as those that were visually visible (greater than 1.0 mm in diameter) or palpable by the surgeon, with a firmer texture than that for normal lymph nodes. Pathological variables comprised tumor type, level of differentiation, and the 8th edition of the American Joint Committee on Cancer tumor, node, metastasis (AJCC TNM) stage. Postoperative data comprised length of hospital stay, rate of postoperative complications (postpancreatectomy hemorrhage (PPH), 13 clinically-relevant postoperative pancreatic fistula (CR-POPF), 14 bile leakage (BL), 15 delayed gastric emptying (DGE), 16 abdominal and wound infection), 17 the use of adjuvant chemotherapy, and reoperation. Details of postoperative tumor recurrence are described in the section titled “Follow-up”.
Follow-up
Patients were followed-up by telephone and outpatient evaluation after discharge. Follow-up evaluation comprised related laboratory tests and imaging examinations. Abdominal enhanced computed tomography and/or magnetic resonance imaging were performed to evaluate tumor metastasis and recurrence. Disease-free survival (DFS) was defined as the duration from the day of surgery until the day of recurrence or the last follow-up. The last follow-up was in March 2023.
Statistical analyses
For normally distributed continuous variables for the whole cohort, the mean ± standard deviation (SD) was calculated, and Student’s t-test was used to assess differences between the groups. Otherwise, the median and interquartile range (IQR) were calculated, and groups were compared using the Mann–Whitney U test. Categorical variables were described as frequency (%) and were analyzed using the chi-square test or Fisher’s exact test. Survival analyses were performed using the Kaplan–Meier method with log-rank tests. Univariable and multivariate Cox regression analyses were used to identify independent risk factors for DFS. All statistical analyses were performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). Considering the small sample size and our clinical experience, we included variables with P < 0.20 in the univariate regression analysis in the multivariate Cox regression analyses. Propensity scores were calculated using logistic regression, and 1:1 patient matching was performed using the nearest-neighbor matching method without replacement. The caliper width was set at 0.2 of the standard deviation of the propensity score logit. Variables included in the matching model were those included as preoperative baseline characteristics. P < 0.05 was considered statistically significant.
Results
Preoperative baseline characteristics
A total of 124 PD patients met the screening criteria, namely, 29 patients who underwent LPD and 95 patients who underwent OPD. After 1:1 optimal matching, 23 patients were successfully matched, and the baseline characteristics after matching are shown in Table 1. There was no significant difference in baseline characteristics between the LPD and OPD groups after matching, among which age, CA199, ALP, and γ-GGT were well-matched.
Table 1.
Baseline characteristics after PSM in the LPD and OPD groups.
| Characteristic | LPD group | OPD group | P |
|---|---|---|---|
| Age (years, mean ± SD) | 66.43 ± 11.48 | 67.70 ± 6.48 | 0.649 |
| Sex (cases (%)) | |||
| male | 14 (60.9) | 17 (73.9) | 0.345 |
| female | 9 (39.1) | 6 (26.1) | |
| BMI (kg/m2) | 21.62 ± 2.37 | 22.98 ± 3.62 | 0.140 |
| CA199 (cases (%)) | |||
| ≤37 kU/L | 9 (39.1) | 10 (43.5) | 0.765 |
| >37 kU/L | 14 (60.9) | 13 (56.5) | |
| ALP (cases (%)) | |||
| ≤125 U/L | 4 (17.4) | 3 (13.0) | 0.681 |
| >125 U/L | 19 (82.6) | 20 (87.0) | |
| γ-GGT (cases (%)) | |||
| ≤50 U/L | 4 (17.4) | 3 (13.0) | 0.681 |
| >50 U/L | 19 (82.6) | 20 (87.0) | |
| DBIL/TBIL (cases (%)) | |||
| <0.6 | 12 (52.2) | 10 (43.5) | 0.555 |
| ≥0.6 | 11 (47.8) | 13 (56.5) | |
| Preoperative biliary drainage (cases (%)) | |||
| Yes | 4 (17.4) | 6 (26.1) | 0.475 |
| No | 19 (82.6) | 17 (73.9) |
PSM, propensity score matching; LPD, laparoscopic pancreaticoduodenectomy; OPD, open laparoscopic pancreaticoduodenectomy; SD, standard deviation; BMI, body mass index; CA199, carbohydrate antigen 199; ALP, alkaline phosphatase; γ-GGT, γ-glutamyl transpeptidase; DBIL/TBIL, direct bilirubin-to-total bilirubin ratio.
Intraoperative characteristics
The intraoperative characteristics of the patients in the LPD and OPD groups are shown in Table 2. Compared with OPD, respectively, LPD was associated with a significantly longer operative time (450.4 minutes vs 391.7 minutes; P = 0.035), lower estimated blood loss volume (100.0 mL vs 200.0 mL; P = 0.042), and shorter tumor diameter (2 cm vs 3 cm; P = 0.003). There was no significant difference between the groups for other intraoperative indicators, such as surgical margin, lymph node enlargement, and tumor invasion into the superior mesenteric vein.
Table 2.
Intraoperative characteristics in the LPD and OPD groups.
| Characteristic | LPD group | OPD group | P |
|---|---|---|---|
| Operative time (minutes, mean ± SD) | 450.39 ± 96.96 | 391.74 ± 85.32 | 0.035 |
| Estimated blood loss volume (mL, median (IQR)) | 100 (100, 285) | 200 (200, 300) | 0.042 |
| R0 resection (cases (%)) | 22 (95.7) | 20 (87.0) | 0.601 |
| Lymph node enlargement (cases (%)) | 12 (52.2) | 18 (78.3) | 0.063 |
| Tumor diameter (cm, median (IQR)) | 2 (2, 2.5) | 3 (2, 3.5) | 0.003 |
| Invasion of the superior mesenteric vein (cases (%)) | 2 (8.7) | 4 (17.4) | 0.662 |
LPD, laparoscopic pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy; SD, standard deviation; IQR, interquartile range.
Pathological and postoperative data
Pathological and postoperative data are shown in Table 3 and Table 4, respectively. Regarding the pathological type, degree of differentiation, and tumor stage, the LPD and OPD groups were similar, with no statistically significant difference. The results showed that patients in the LPD group had shorter hospital stays (16.4 days vs 20.4 days) and higher rates of postoperative complications (91.3% vs 73.9%), compared with the OPD group; however, there was no statistically significant difference. Other indicators, such as reoperation and postoperative adjuvant chemotherapy, were similar between the two groups.
Table 3.
Pathological characteristics in the LPD and OPD groups.
| Characteristic | LPD group | OPD group | P |
|---|---|---|---|
| Pathological type (cases (%)) | |||
| Pancreatic carcinoma | 11 (47.8) | 12 (52.2) | 0.633 |
| Distal bile duct carcinoma | 4 (17.4) | 4 (17.4) | |
| Duodenal carcinoma | 4 (17.4) | 6 (26.1) | |
| Ampullary carcinoma | 4 (17.4) | 1 (4.3) | |
| Degree of differentiation (cases (%)) | |||
| High | 4 (17.4) | 2 (8.7) | 0.363 |
| Moderate | 7 (30.4) | 3 (13.0) | |
| High-to-moderate | 2 (8.7) | 6 (26.1) | |
| Moderate-to-low | 8 (34.8) | 10 (43.5) | |
| Unknown | 2 (8.7) | 2 (8.7) | |
| AJCC TNM stage (cases (%)) | |||
| IA | 4 (17.4) | 2 (8.7) | 0.579 |
| IB | 5 (21.7) | 8 (34.8) | |
| IIA | 4 (17.4) | 6 (26.1) | |
| IIB | 10 (43.5) | 7 (30.4) |
LPD, laparoscopic pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy; AJCC TNM stage, American Joint Committee on Cancer staging system (tumor, node, metastasis) 8th edition.
Table 4.
Postoperative outcomes in the LPD and OPD groups.
| Characteristic | LPD group | OPD group | P |
|---|---|---|---|
| Postoperative length of stay (days, mean±SD) | 16.35 ± 4.74 | 20.35 ± 12.47 | 0.162 |
| PPH | 3 (13.0) | 3 (13.0) | 1.000 |
| CR-POPF | 3 (13.0) | 5 (21.7) | 0.697 |
| BL | 6 (26.1) | 2 (8.7) | 0.243 |
| Intra-abdominal infection | 4 (17.4) | 5 (21.7) | 1.000 |
| DGE | 2 (8.7) | 2 (8.7) | 1.000 |
| Wound infection | 3 (13.0) | 0 (0.0) | 0.232 |
| Overall complications | 21 (91.3) | 17 (73.9) | 0.243 |
| Reoperation | 1 (4.3) | 2 (8.7) | 1.000 |
| Adjuvant chemotherapy | 14 (60.9) | 14 (60.9) | 1.000 |
| Recurrence | 17 (73.9) | 16 (69.6) | 0.743 |
Values are cases (%) unless otherwise stated.
LPD, laparoscopic pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy; PPH, postpancreatectomy hemorrhage; CR-POPF clinically-relevant postoperative pancreatic fistula; BL, biliary leakage; DGE, delayed gastric emptying.
DFS and prognostic factors
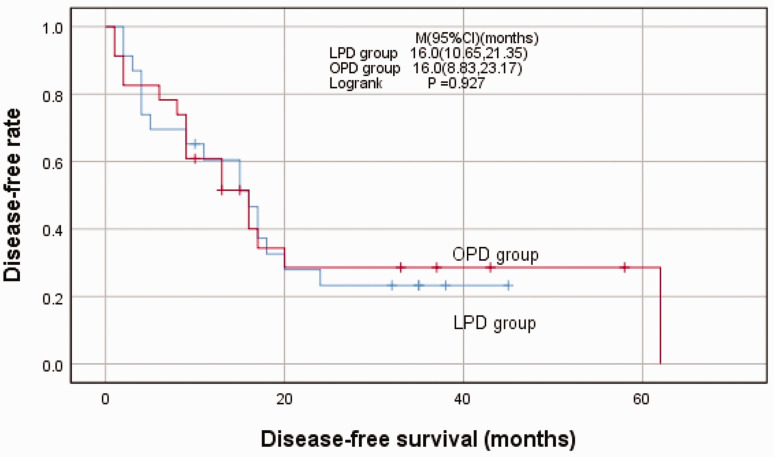
By the end of the follow-up, 17 patients (73.9%) in the LPD group and 16 patients (69.6%) in the OPD group had relapsed, with no significant difference. The median follow-up time was 15 months and 13 months in the LPD group and OPD group, respectively. No significant difference was detected regarding the median DFS in the groups (LPD vs OPD, 16.0 months vs 16.0 months; Figure 2).
Figure 2.
Kaplan–Meier curves for disease-free survival in patients who underwent OPD and LPD
LPD, laparoscopic pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy.
Univariate Cox regression analysis (Table 5) showed that the level of ALP and γ-GGT (P = 0.127), tumor differentiation (P = 0.053), surgical margin (P = 0.066), tumor diameter (P = 0.003), lymph node enlargement (P = 0.007), and adjuvant treatment (P = 0.151) were associated with DFS in both groups. However, only low levels of ALP and γ-GGT (hazard ratio (HR) = 0.202, 95% confidence interval (CI) = 0.055–0.750; P = 0.017), positive surgical margin (HR = 4.692, 95% CI = 1.101–19.991; P = 0.037), and lymph node enlargement (HR = 2.868, 95% CI = 1.071–7.683; P = 0.036) were validated as independent risk factors by multivariate analysis (Table 5).
Table 5.
Univariate and multivariate Cox regression analyses to identify the predictors of DFS (n = 46) in patients with VPC.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Age (years) | 1.002 (0.966, 1.040) | 0.917 | ||
| Sex (Male vs female) | 0.900 (0.425, 1.907) | 0.784 | ||
| BMI | 0.992 (0.876, 1.123) | 0.896 | ||
| DBIL/TBIL(>0.6 vs ≤0.6) | 0.674 (0.336, 1.353) | 0.267 | ||
| ALP (U/L) (>125 vs ≤125) | 0.498 (0.203, 1.218) | 0.127 | 0.202 (0.055, 0.750) | 0.017 |
| γ-GGT (U/L) (>50 vs ≤50) | 0.498 (0.203, 1.218) | 0.127 | 0.202 (0.055, 0.750) | 0.017 |
| CA199 (kU/L)(>37 vs ≤37) | 2.081 (0.999, 4.335) | 0.050 | 2.032 (0.894, 4.725) | 0.100 |
| Biliary drainage (Yes vs No) | 0.965 (0.369, 2.518) | 0.941 | ||
| Differentiation | ||||
| High | Reference | |||
| High-to-moderate | 1.681 (0.401, 7.045) | 0.477 | 0.659 (0.138, 3.149) | 0.602 |
| Moderate | 1.756 (0.437, 7.062) | 0.428 | 1.755 (0.388, 7.947) | 0.465 |
| Moderate-to-low | 3.426 (0.984, 11.924) | 0.053 | 2.342 (0.584, 9.385) | 0.230 |
| Surgical margin (R1 vs R0) | 3.169 (0.927, 10.831) | 0.066 | 4.692 (1.101, 19.991) | 0.037 |
| AJCC TNM stage | ||||
| IA | Reference | |||
| IB | 0.591 (0.187, 1.865) | 0.370 | ||
| IIA | 0.736 (0.233, 2.324) | 0.601 | ||
| IIB | 1.290 (0.457, 3.642) | 0.631 | ||
| Tumor diameter (cm) | 1.756 (1.208, 2.552) | 0.003 | 1.571 (0.887, 2.592) | 0.128 |
| Invasion of the SMV (Yes vs No) | 1.506 (0.521, 4.355) | 0.449 | ||
| Lymph node enlargement (Yes vs No) | 3.092 (1.363, 7.011) | 0.007 | 2.868 (1.071, 7.683) | 0.036 |
| Operative time (minutes) | 1.002 (0.998, 1.005) | 0.379 | ||
| Estimated blood loss volume (mL) | 1.001 (0.999, 1.002) | 0.426 | ||
| Postoperative length of stay (days) | 1.002 (0.968, 1.037) | 0.922 | ||
| PPH (Yes vs No) | 1.278 (0.443, 3.685) | 0.650 | ||
| CR-POPF (Yes vs No) | 0.731 (0.281, 1.901) | 0.520 | ||
| BL (Yes vs No) | 1.664 (0.707, 3.916) | 0.243 | ||
| Wound infection (Yes vs No) | 0.798 (0.190, 3.355) | 0.758 | ||
| DGE (Yes vs No) | 2.238 (0.650, 7.704) | 0.201 | ||
| Abdominal infection (Yes vs No) | 0.865 (0.355, 2.105) | 0.749 | ||
| Reoperation (Yes vs No) | 0.324 (0.044, 2.380) | 0.268 | ||
| Adjuvant chemotherapy (Yes vs No) | 0.599 (0.298, 1.206) | 0.151 | 0.453 (0.191, 1.074) | 0.072 |
| Surgical type (LPD vs OPD) | 0.969 (0.483, 1.944) | 0.930 |
DFS, disease-free survival; VPC, carcinoma of the ampulla of Vater; HR, hazard ratio; CI, confidence interval; BMI, body mass index; DBIL/TBIL, direct bilirubin-to-total bilirubin ratio; ALP, alkaline phosphatase; γ-GGT, γ-glutamyl transpeptidase; CA199, carbohydrate antigen 199; AJCC TNM stage, American Joint Committee on Cancer staging system (tumor, node, metastasis), 8th edition; SMV, superior mesenteric vein; PPH, postpancreatectomy hemorrhage; CR-POPF, clinically-relevant postoperative pancreatic fistula; BL, biliary leakage; DGE, delayed gastric emptying; LPD, laparoscopic pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy.
Discussion
With recent rapid developments in minimally invasive surgery, this type of surgery, such as laparoscopic hepatectomy, laparoscopic distal pancreatectomy, and laparoscopic radical gastrectomy, has matured clinically, and its advantages are widely recognized.5,18–20 However, progress in LPD has been slow. The first LPD was performed by Gagner in 1994. 21 Because the pancreas is adjacent to extremely important visceral and vascular structures, pancreatic operation time is long, and postoperative complications are frequent and serious, LPD can be called the “Everest” of general surgery.3,4 Recently, there have been numerous studies of the efficacy of LPD, and its advantages, such as less intraoperative bleeding and shorter hospital stay, have been confirmed.7–9,22–24 However, the safety and postoperative survival rate of LPD remain controversial. The LEOPARD-2 trial reported a higher 90-day complication-related death rate in the LPD vs OPD groups, 25 while another multicenter randomized controlled trial confirmed that LPD was safe and effective in patients with pancreatic or periampullary tumors when performed by experienced pancreatic surgeons. 26 However, these studies focused mainly on high-volume centers with mature technology. There are almost no such studies in medium-volume medical centers, which is not in accordance with the actual situation for most hospitals. PSM analysis is an effective strategy to reduce the effects of confounding factors in observational studies. 27 Recently, scholars in Japan and other countries have focused on PSM in comparisons of LPD and OPD and have reported initial results.24,28,29 Therefore, we selected PSM analysis to compare the efficacy of LPD and OPD in a medium-volume medical center.
Our study found that intraoperative blood loss volume in the LPD group was less than that in the OPD group, and the difference was statistically significant (P = 0.042), similar to findings in most published studies in large-volume centers.22–24,26,28,29 This could have resulted from the magnified visibility during laparoscopy and minimal disruption to tissues.28,30 The longer operative time in the LPD group could be explained by the different length of the learning curve. Our institution is at the technical competence stage. 31 As for blood loos volume, intraoperative tumor diameter was smaller in the LPD vs OPD groups. Serrano et al. 32 concluded that margin status was independently associated with the overall survival of individuals with pancreatic carcinoma. In our study, there was a higher R0 resection rate in the LPD group, but the rate was not significantly higher than that in the OPD group. This finding was similar to the result of a PSM analysis performed by Zhou et al. in pancreatic ductal adenocarcinoma. 24 There was no significant difference in oncological outcomes between the groups.
The postoperative complication rate is an important indicator to evaluate the safety and efficacy of surgery. In this study, the total incidence of complications in the LPD group was 91.3%, which was higher than the incidence of 73.9% in the OPD group. However, there was no statistically significant difference between the groups. The high postoperative complication rate with LPD may be related to the long and steep learning curve.25,33 PPH is the most urgent and potentially life-threatening complication after PD.3,4,6 In our study, the incidence of postoperative bleeding was 13.0% in both groups, which was consistent with a large multicenter retrospective analysis in our country. 34 In the LPD group, three cases of postoperative bleeding, 1 case from the superior mesenteric artery and two cases from the gastrointestinal anastomosis, improved after vascular interventional embolization or endoscopic hemostasis. POPF is the most common complication after PD, and it has been a major problem perplexing pancreatic surgeons worldwide for many years.3,4,6,35 The present study showed that the incidence of CR-POPF in the LPD group was 13.0%, which was lower than that in the OPD group (21.7%), although there was no significant difference between the groups. A recently published study using the CUSUM analysis reported that the incidence of grade B/C pancreatic fistula decreased to 20% during the third period of the learning curve. 31 Our results did not approach the results in reports from most high-volume centers, even the National Cancer Data Base, 36 which reported stable rates of pancreatic fistula of <10%. In the first few years, our center adopted the traditional method of duct-to-mucosa anastomosis, and then gradually introduced “Hong’s pancreaticojejunostomy” method. 37 The latter method involves placing a drain between the pancreatic stump and the jejunum to create an “artificial internal fistula” for internal drainage of pancreatic fluid. After introducing this method, the incidence of pancreatic fistula decreased, and the pancreaticojejunostomy time decreased to 30 minutes in our center. Zhou et al. 24 reported a higher rate of wound infection in the LPD group vs the OPD group; however, no significant difference was observed. In the LPD group, one patient underwent reoperation and resuture to repair skin suture failure. There were two reoperations in the OPD group, one of which was owing to skin suture split; the other patient underwent partial gastrectomy owing to gastrointestinal anastomotic bleeding.
Regarding postoperative survival, Kaplan–Meier survival analyses showed that median DFS in the two groups was the same (16.0 months vs 16.0 months, respectively), similar to results in previous reports26,28 that also confirmed the safety and feasibility of LPD in medium-volume centers. In the multivariate regression analysis, we found that low levels of ALP and γ-GGT, positive surgical margin, and intraoperative lymph node enlargement were independent risk factors for DFS in VPC patients; the latter two prognostic variables have been confirmed in many studies.24,28,32 Interestingly, ALP level >125 U/L and γ-GGT level >50 U/L were identified as protective factors for postoperative recurrence. Most previously published studies discussed the correlation between CA199 values and the prognosis of patients with pancreatic cancer or VPC.38,39 There are few studies reflecting the relationship between the degree of tumor obstruction and prognosis. Similar to our study, in a retrospective study, Huang et al. 40 concluded that preoperative combined calculation of CA199/ALP and CA199/γ-GGT ratios could effectively predict the prognosis of patients with pancreatic cancer. The authors found significant survival benefits in pancreatic cancer patients with CA199/ALP <0.7 (HR = 2.600, 95% CI = 1.509–4.479; P < 0.001) and CA19-9/γ-GGT <0.4 (HR = 2.410, 95% CI = 1.410–4.120; P = 0.001). However, the relationship between ALP and γ-GGT and DFS in patients with periampullary carcinoma has not been fully confirmed. We predict that ALP and γ-GGT will become indicators to assess the extent of tumor invasion. Patients with high ALP and γ-GGT levels may have a tendency to seek earlier healthcare, partly contributing to the increase in the number of patients who receive timely treatment. This provides a novel direction for future large-scale randomized controlled trials.
Our center began to perform LPD in 2016, and to date, the number of LPD operations has exceeded 100. We are currently transitioning from the second period to the third period of the learning curve.31,41 We also regularly arrange for pancreatic surgeons who are qualified in LPD to study laparoscopic techniques in high-volume centers in Japan and internationally. Additionally, all surgeons are proficient in performing OPD before performing LPD.
Notably, a recent meta-analysis concluded that LPD showed no advantage over OPD and considered that currently, LPD could not be proposed as an equivalent alternative to OPD. 42 However, on the basis of the above analysis of these two different surgical approaches, LPD achieves excellent outcomes in terms of surgical margin status, intraoperative blood loss volume, and postoperative hospital stay. Furthermore, the prognosis of patients in the LPD group was not inferior to that of the OPD group, in our study, and the results were similar to those reported by large-volume centers in Japan and internationally.8,9,24,26,28,36,43,44 However, the gap between large- and moderate-volume centers mainly involves lymph node dissection and postoperative complication rates. The reasons for these differences are as follows: First, patients who undergo LPD are usually in the early stage of the disease, and the degree of differentiation is mainly high or moderate. For patients with advanced-stage cancer, we evaluate cautiously to choose the surgical approach. When the operation is uncertain, conversion to open surgery is often necessary. Second, our current experience with digestive tract reconstruction is insufficient. Pancreaticojejunostomy is still based mainly on pancreatic duct-to-mucosa anastomosis. However, our institution requires more skill to master the technique, and there is the possibility of unstable alignment, residual dead space, and suture loss. Third, postoperative patient management is not standardized. For example, postoperative patients are not routinely followed by abdominal contrast-enhanced computed tomography or computed tomography angiography, except when the patient has obvious symptoms. Therefore, we believe that through the improvement of preoperative, intraoperative, and postoperative management, the efficacy of LPD in medium-volume pancreatic centers can approach and even reach the level of high-volume centers.
Our study has several limitations: First, this was a single-center retrospective study with a small sample size. Second, the follow-up time was relatively short. Third, bias was present in the selection of the included indicators for PSM. Moreover, other factors with a significant impact on prognosis were not included in the PSM. Last but not least, the relationship between ALP and γ-GGT and the prognosis of periampullary carcinoma remains to be confirmed because of the limited number of studies. Large-scale randomized controlled studies in multiple medium-volume centers are needed.
Conclusions
LPD in medium-volume centers with acceptable technical conditions may approach or even achieve the efficacy of LPD in large-volume centers. However, qualified cases should be selected carefully, and the operation should be performed by experienced surgeons.
Acknowledgements
We wish to thank the medical staff of the Department of Hepatobiliary and Pancreatic Surgery of Shaoxing People’s Hospital for their generous help.
Author contributions: Chenming Liu and Yuxing Liu designed the study and wrote the manuscript. Jiaming Dong performed the literature search and data analysis. Yingjie Chai performed the initial manuscript revision. Haijun Tang critically revised the manuscript and provided final approval. Chenming Liu, Yuxing Liu, and Jiaming Dong contributed equally to this work. All authors have read the manuscript and approve its contents.
The Authors declare that there is no conflict of interest. The copyright transfer statement and submission letter have been signed by all authors.
Funding: This work was supported by the Shaoxing Basic Public Welfare Project [grant number 2022A14012] and the Shaoxing Health Science and Technology Project (laboratory opening plan) [grant number 2022SY013].
ORCID iDs: Chenming Liu https://orcid.org/0009-0003-3622-0400
Yuxing Liu https://orcid.org/0009-0008-7564-0092
Data availability statement
The data supporting the conclusions in this study are available from the corresponding author on reasonable request.
References
- 1.Hayes DH, Bolton JS, Willis GW, et al. Carcinoma of the ampulla of Vater. Ann Surg 1987; 206: 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Rooij T, Tol JA, Van Eijck CH, et al. Outcomes of distal pancreatectomy for pancreatic ductal adenocarcinoma in the Netherlands: a nationwide retrospective analysis. Ann Surg 2016; 23: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppola A, Stauffer JA, Asbun HJ. Laparoscopic pancreatoduodenectomy: current status and future directions. Updates Surg 2016; 68: 217–224. [DOI] [PubMed] [Google Scholar]
- 4.Qin R, Kendrick ML, Wolfgang CL, et al. International expert consensus on laparoscopic pancreaticoduodenectomy. Hepatobiliary Surg Nutr 2020; 9: 464–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Rooij T, Klompmaker S, Abu Hilal M, et al. Laparoscopic pancreatic surgery for benign and malignant disease. Nat Rev Gastroenterol Hepatol 2016; 13: 227–238. [DOI] [PubMed] [Google Scholar]
- 6.Boggi U, Amorese G, Vistoli F, et al. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 2015; 29: 9–23. [DOI] [PubMed] [Google Scholar]
- 7.Nassour I, Wang SC, Christie A, et al. Minimally invasive versus open pancreaticoduodenectomy: a propensity-matched study from a national cohort of patients. Ann Surg 2018; 268: 151–157. [DOI] [PubMed] [Google Scholar]
- 8.Yin T, Qin T, Wei K, et al. Comparison of safety and effectiveness between laparoscopic and open pancreatoduodenectomy: a systematic review and meta-analysis. Int J Surg 2022; 105: 106799. [DOI] [PubMed] [Google Scholar]
- 9.Yan Y, Hua Y, Chang C, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic and periampullary tumor: a meta-analysis of randomized controlled trials and non-randomized comparative studies. Front Oncol 2023; 12: 1093395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tempero MA. NCCN Guidelines updates: pancreatic cancer. J Natl Compr Canc Netw 2019; 17: 603–605. [DOI] [PubMed] [Google Scholar]
- 11.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 12.Study Group of Pancreatic Surgery in Chinese Society of Surgery of Chinese Medical Association, Pancreas of Minimally Invasive Treatment Group in Pancreatic Disease Branch of China International Exchange and Promotion Association for Medical and Healthcare, Pancreas Minimally Invasive Group in Pancreatic Diseases Committee of Chinese Research Hospital Association, Pancreas Minimally Invasive Group in Pancreatic Cancer Committee of Chinese Anti-Cancer Association. [Expert consensus of laparoscopic pancreaticoduodenectomy.] Chin J Surg 2017; 55: 335–339.28464571 [Google Scholar]
- 13.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007; 142: 20–25. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017; 161: 584–591. [DOI] [PubMed] [Google Scholar]
- 15.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011; 149: 680–688. [DOI] [PubMed] [Google Scholar]
- 16.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007; 142: 761–768. [DOI] [PubMed] [Google Scholar]
- 17.Study Group of Pancreatic Surgery in Chinese Society of Surgery of Chinese Medical Association, Pancreatic Disease Committee of Chinese Research Hospital Association, Editorial Board of Chinese Journal of Surgery. [A consensus statement on the diagnosis, treatment and prevention of common complications after pancreatic surgery.] Chin J Surg 2017; 55: 328–334. [DOI] [PubMed] [Google Scholar]
- 18.Maki H, Hasegawa K. Advances in the surgical treatment of liver cancer. Biosci Trends 2022; 16: 178–188. [DOI] [PubMed] [Google Scholar]
- 19.Cucchetti A, Bocchino A, Crippa S, et al. Advantages of laparoscopic distal pancreatectomy: systematic review and meta-analysis of randomized and matched studies. Surgery 2022; 1023–1029. [DOI] [PubMed] [Google Scholar]
- 20.Lou S, Yin X, Wang Y, et al. Laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Int J Surg 2022; 102: 106678. [DOI] [PubMed] [Google Scholar]
- 21.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994; 8: 408–410. [DOI] [PubMed] [Google Scholar]
- 22.Khaled YS, Fatania K, Barrie J, et al. Matched case-control comparative study of laparoscopic versus open pancreaticoduodenectomy for malignant lesions. Surg Laparosc Endosc Percutan Tech 2018; 28: 47–51. [DOI] [PubMed] [Google Scholar]
- 23.Shin SH, Kim YJ, Song KB, et al. Totally laparoscopic or robot-assisted pancreaticoduodenectomy versus open surgery for periampullary neoplasms: separate systematic reviews and meta-analyses. Surg Endosc 2017; 31: 3459–3474. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Jin W, Wang D, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a propensity score matching analysis. Cancer Commun (Lond) 2019; 39: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Hilst J, De Rooij T, Bosscha K, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019; 4: 199–207. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Li D, Chen R, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2021; 6: 438–447. [DOI] [PubMed] [Google Scholar]
- 27.Yao XI, Wang X, Speicher PJ, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 2017; 109: djw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Yin T, Qin T, et al. Comparison of laparoscopic versus open pancreaticoduodenectomy in patients with resectable pancreatic ductal adenocarcinoma: a propensity score-matching analysis of long-term survival. Pancreatology 2022; 22: 317–324. [DOI] [PubMed] [Google Scholar]
- 29.Lee CS, Kim EY, You YK, et al. Perioperative outcomes of laparoscopic pancreaticoduodenectomy for benign and borderline malignant periampullary disease compared to open pancreaticoduodenectomy. Langenbecks Arch Surg 2018; 403: 591–597. [DOI] [PubMed] [Google Scholar]
- 30.Tian F, Wang YZ, Hua SR, et al. Laparoscopic assisted pancreaticoduodenectomy: an important link in the process of transition from open to total laparoscopic pancreaticoduodenectomy. BMC Surg 2020; 20: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dokmak S, Aussilhou B, Ftériche FS, et al. The outcome of laparoscopic pancreatoduodenectomy is improved with patient selection and the learning curve. Surg Endosc 2022; 36: 2070–2080. [DOI] [PubMed] [Google Scholar]
- 32.Serrano PE, Cleary SP, Dhani N, et al. Improved long-term outcomes after resection of pancreatic adenocarcinoma: a comparison between two time periods. Ann Surg Oncol 2015; 22: 1160–1167. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Zhou F, Li L, et al. Analysis of safety of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy: propensity score matching analysis. China J Hepatobiliary Surg 2021; 27: 520–524. [Google Scholar]
- 34.Wang M, Peng B, Liu J, et al. Practice patterns and perioperative outcomes of laparoscopic pancreaticoduodenectomy in China: a retrospective multicenter analysis of 1029 patients. Ann Surg 2021; 273: 145–153. [DOI] [PubMed] [Google Scholar]
- 35.Wang N, Yang J, Pan Y, et al. Comparison of perioperative outcomes between laparoscopic and open pancreaticoduodenectomy: a single-center retrospective study. China J Hepatobiliary Surg 2021; 27: 594–598. [Google Scholar]
- 36.Sharpe SM, Talamonti MS, Wang CE, et al. Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: a comparison of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg 2015; 221: 175–184. [DOI] [PubMed] [Google Scholar]
- 37.Hong D, Li H, Liu X, et al. Incidence of postoperative pancreatic fistula after using a defined pancreaticojejunostomy technique for laparoscopic pancreaticoduodenectomy: a prospective multicenter study on 1033 patients. Int J Surg 2022; 101: 106620. [DOI] [PubMed] [Google Scholar]
- 38.Luo G, Jin K, Deng S, et al. Roles of CA19-9 in pancreatic cancer: biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer 2021; 1875: 188409. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka H, Tamura K, Abe T, et al. Serum carboxypeptidase activity and genotype-stratified CA19-9 to detect early-stage pancreatic cancer. Clin Gastroenterol Hepatol 2022; 20: 2267–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang X, Lu Z, Zhang K, et al. Prognostic impact of the ratio of preoperative CA19-9 to liver enzyme levels in pancreatic cancer patients with jaundice (predictability of combined CA19-9/AST and CA19-9/γ-GGT for jaundiced PDAC patients). Pancreatology 2021; S1424-3903: 00470–1. [DOI] [PubMed] [Google Scholar]
- 41.Choi M, Hwang HK, Lee WJ, et al. Total laparoscopic pancreaticoduodenectomy in patients with periampullary tumors: a learning curve analysis. Surg Endosc 2021; 35: 2636–2644. [DOI] [PubMed] [Google Scholar]
- 42.Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic versus open pancreaticoduodenectomy: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 2020; 271: 54–66. [DOI] [PubMed] [Google Scholar]
- 43.Chen K, Zhou Y, Jin W, et al. Laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic outcomes and long-term survival. Surg Endosc 2020; 34: 1948–1958. [DOI] [PubMed] [Google Scholar]
- 44.Kantor O, Talamonti MS, Sharpe S, et al. Laparoscopic pancreaticoduodenectomy for adenocarcinoma provides short-term oncologic outcomes and long-term overall survival rates similar to those for open pancreaticoduodenectomy. Am J Surg 2017; 213: 512–515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions in this study are available from the corresponding author on reasonable request.