Figure 3.
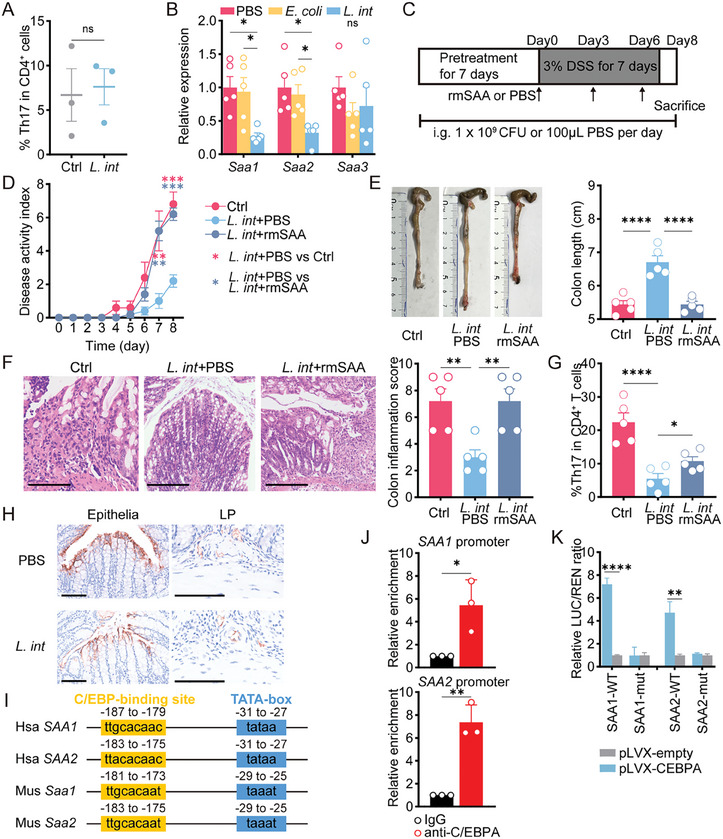
L. intestinalis suppressed Th17 cell differentiation by targeting epithelial SAA production. A) In vitro Th17 differentiation assay was performed on naïve CD4+ T cells isolated from mouse spleen and treated with PBS (Ctrl) or L. intestinalis (L. int) (n = 3). B) Expression of Saa1/2/3 was evaluated in bulk colon samples from chronic DSS‐treated mice with gavage of PBS, E. coli, or L. intestinalis (L. int) (n = 5). C) Pretreatment with gavage of PBS or L. intestinalis (L. int) was started on the 7th day before DSS treatment and until the end of the experiment. On days zero, three, and six of DSS course, all mice were intraperitoneally injected (i.p.) with recombinant mouse SAA proteins (rmSAA) or PBS. D–G) The pathology of colitis was evaluated among PBS‐i.p. mice with PBS(PBS+PBS) or L. intestinalis (L. int+PBS) gavage, as well as rmSAA‐i.p. mice with L. intestinalis (L. int+rmSAA) gavage, by disease activity index (D), colon length (E), pathological scores (scale bar, 200 µm) (F), and frequencies of Th17 cells in colon LP (G) (n = 5). H) Representative fluorescent immunohistochemistry images indicated the expression of Saa1/2 in colon epithelia and LP from chronic DSS‐treated mice with gavage of PBS and L. intestinalis (L. int). I) The C/EBP‐binding motifs were conservative among the sequences of promoter regions from human (Hsa) SAA1/2 genes or mouse (Mus) Saa1/2 genes. J) The enrichment at SAA1 and SAA2 promoter was detected by ChIP‐qPCR in C/EBPA‐overexpressing HEK‐293T cells using anti‐C/EBPA or control IgG. K) The firefly luciferase (LUC) was driven by wildtype (WT) promoter or mutate (mut) regions of SAA1 and SAA2. The trans‐activation ability of C/EBPA was measured by the LUC / REN ratio in HEK‐293T cells co‐transfected with pLVX‐empty or pLVX‐CEBPA. Error bars indicate mean ± SEM. ns, no significance; * p < 0.05; ** p < 0.01; **** p < 0.0001. p values were based on Student's t‐test and one‐way ANOVA with post‐hoc test.
