Figure 4.
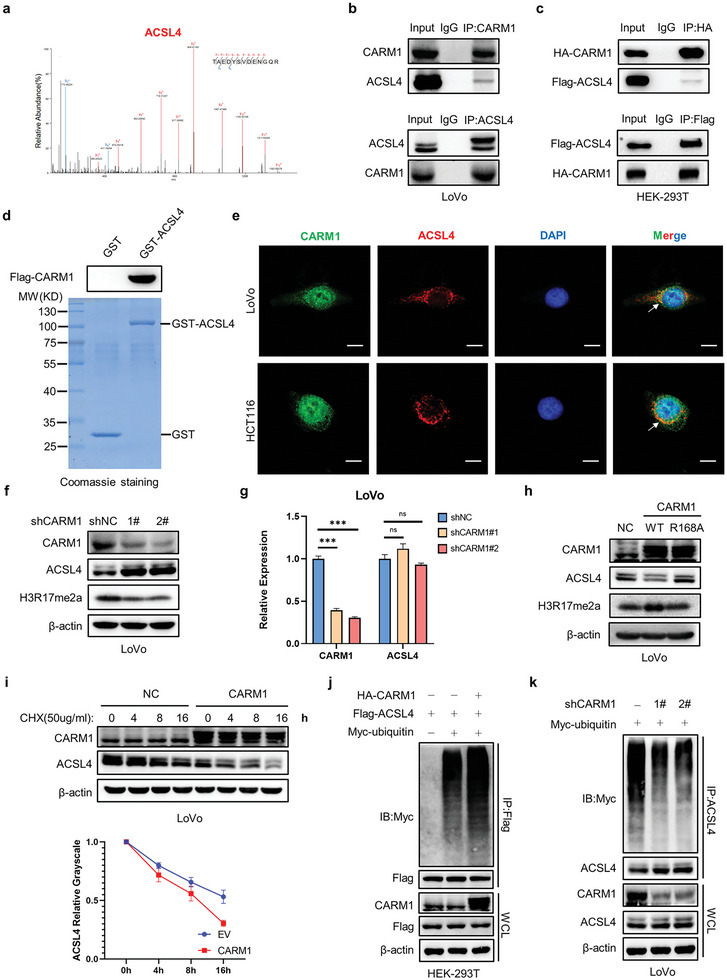
CARM1 directly interacts with and decreases ACSL4 protein levels in colon cancer cells. a) Mass spectrometry analysis identified ACSL4 in the binding protein pool of CARM1. b) Immunoprecipitation (IP) analyses were performed to examine the endogenous interaction between CARM1 and ACSL4 by using antibodies against CARM1 and ACSL4 in LoVo cells. c) IP analyses were performed to examine the exogenous interaction between CARM1 and ACSL4 by using antibodies against Flag and HA, respectively, in HEK293T cells. d) In vitro GST pull‐down assay to verify the binding of CARM1 and ACSL4. e) Immunofluorescence staining was performed to observe the colocalization of CARM1 (green) and ACSL4 (red) in LoVo and HCT116 cells. The nucleus is labeled via DAPI (blue). Scale bar, 20 µm. f) Western blot analysis of the indicated LoVo cells. Protein levels of CARM1, ACSL4, and H3R17me2a were assayed. g) Quantitative real‐time PCR (qPCR) analysis of CARM1 and ACSL4 mRNA levels in the indicated LoVo cells (n = 3 independent experiments). h) Western blot analysis of the indicated LoVo cells. Protein levels of CARM1, ACSL4, and H3R17me2a were assayed. i) Western blot analysis of vector‐ and CARM1‐overexpressing LoVo cells treated with 50 µg mL−1 cycloheximide for the indicated times. Quantitative analysis was conducted on ACSL4 levels at the indicated time points. j) IP with an anti‐Flag antibody and Western blotting with an anti‐Myc antibody were performed to detect the ubiquitination level of ACSL4. k) CARM1‐knockdown cells were transfected with the indicated plasmid and treated with MG132 (10 × 10−6 m) for 8 h. IP with an anti‐ACSL4 antibody and Western blot analysis of the ubiquitination of endogenous ACSL4 were performed. The data shown represent the mean ± SD. Comparisons were made by using one‐way ANOVA with Tukey's test; *** p < 0.001; n.s., no significant difference.
