Figure 6.
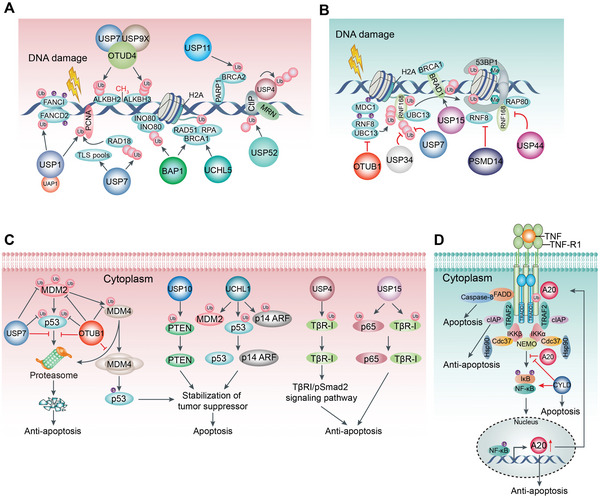
DUBs play multi‐faced roles in tumor‐associated DNA repair and apoptosis. A) Replication‐associated repair is regulated by DUBs. USP1 promotes multiple rounds of repair in the S phase by modulating the turnover of FANCD2‐FANCI monoubiquitination at the damaged sites. Similarly, PCNA is deubiquitinated by USP1 to govern the error‐prone translesion synthesis repair pathway. USP7 indirectly coordinates PCNA deubiquitination. OTUD4 acts as a scaffold to form a complex with USP7 and USP9X which interacts with ALKBH2 and ALKBH3 to suppress their ubiquitination and degradation. ALKBH2 and ALKBH3 preferentially remove methyl groups from single‐stranded and double‐stranded DNA and reverse DNA alkylation. In addition, USP9X upholds the stability of the DNA replication fork and the response of DNA damage checkpoint by modulating the protein claspin in the S phase. BAP1 promotes HR repair by recruiting BRCA1, RAD51, and RPA to DSB sites and reduces ubiquitinated forms of H2A and H2AX. USP11 forms a complex with BRCA2 that boosts HR in DSB sites and limits PARP1 activity. DUBs govern Ub‐dependent signaling, contributing to DNA repair. USP4 autodeubiquitylation regulates CtIP recruitment to sites of DNA damage. USP52 deubiquitinates CtIP, thereby promoting and facilitating the phosphorylation and activation of CtIP for DNA repair. B) Double‐strand breaks (DSBs) promote ATM kinase to phosphorylate histone H2A and MDC1, a mediator of DNA damage checkpoint protein 1. This leads to the recruitment of E3 ligase RNF8 conjugated with E2 enzyme UBC13 that induces the production of Lys63‐linked Ub chains on scaffold proteins (such as RNF168) in response to DNA damage. Several DUBs counteract this ubiquitination in diverse manners including OTUB1, USP34, and USP7, although some are functionally redundant. Additionally, oligomerized 53BP1 is stably recruited by RNF168‐ and RNF8‐regulated chromatin ubiquitination, which is inhibited by PSMD14 and USP44. USP15 deubiquitinates BARD1, and promotes BARD1‐HP1γ interaction, resulting in BRCA1/BARD1 retention at DSBs. C) The role of DUBs in regulating apoptosis. DUBs increase or decrease apoptosis by inhibiting proteasomal degradation of pro‐apoptotic or anti‐apoptotic proteins through deubiquitination. USP7, OTUB1, UCHL1, and USP10 promote apoptosis by enhancing the stability of tumor suppressors such as p53, PTEN, and p14 ARF. Conversely, the oncogene of TβR‐I and p65 are stabilized by USP4 and USP15, which exert an anti‐apoptotic effect. D) Moreover, A20 is part of a negative feedback mechanism inhibiting continuous NF‐κB activation during single TNF stimulation, wherein A20 expression is constitutively activated and prevents cells from apoptosis through stabilizing the Ub network involved in the TNFR1 signaling complex. However, the DUB CYLD antagonizes this process.
