Abstract
Background and Hypothesis
Low-grade neural and peripheral inflammation are among the proposed pathophysiological mechanisms of schizophrenia. White matter impairment is one of the more consistent findings in schizophrenia but the underlying mechanism remains obscure. Many cerebral white matter components are sensitive to neuroinflammatory conditions that can result in demyelination, altered oligodendrocyte differentiation, and other changes. We tested the hypothesis that altered immune-inflammatory response system (IRS) and compensatory immune-regulatory reflex system (IRS/CIRS) dynamics are associated with reduced white matter integrity in patients with schizophrenia.
Study Design
Patients with schizophrenia (SCZ, 70M/50F, age = 40.76 ± 13.10) and healthy controls (HCs, 38M/27F, age = 37.48 ± 12.31) underwent neuroimaging and plasma collection. A panel of cytokines were assessed using enzyme-linked immunosorbent assay. White matter integrity was measured by fractional anisotropy (FA) from diffusion tensor imaging using a 3-T Prisma MRI scanner. The cytokines were used to generate 3 composite scores: IRS, CIRS, and IRS/CIRS ratio.
Study Results
The IRS/CIRS ratio in SCZ was significantly higher than that in HCs (P = .009). SCZ had a significantly lower whole-brain white matter average FA (P < .001), and genu of corpus callosum (GCC) was the most affected white matter tract and its FA was significantly associated with IRS/CIRS (r = 0.29, P = .002). FA of GCC was negatively associated with negative symptom scores in SCZ (r = −0.23, P = .016). There was no mediation effect taking FA of GCC as mediator, for that IRS/CIRS was not associated with negative symptom score significantly (P = .217) in SCZ.
Conclusions
Elevated IRS/CIRS might partly account for the severity of negative symptoms through targeting the integrity of GCC.
Keywords: Schizophrenia, IRS/CIRS, white matter, genu of corpus callosum, negative symptom
Introduction
Schizophrenia is a chronic and severe mental illness that is characterized by disturbance of positive and negative symptoms and lower functional status and cognitive deficits. Patients with schizophrenia also show significantly lower integrity of cerebral white matter, in particular in the cerebral tracts that interconnect associated functional areas.1–3 Diffusion tensor imaging (DTI) revelated patients with schizophrenia suffer from specific deficit patterns of microstructural damages that are regionally specific with associative white matter tracts such as genu, body and splenium of corpus callosum, cingulum bundles, cortico-spinal fasciculus, superior longitude fasciculus, and inferior fronto-occipital fasciculus show the biggest deficits.1,4–8 The degree of deficits in these tracts were sometimes found to be correlated with the symptoms of the illness, which included anhedonia, negative symptoms and treatment resistance.1,6 However, the pathophysiology mechanism underlying the relationship of reduced white matter integrity remains unclear.
These white matter deficits are observed in first-episode patients with schizophrenia (FEPS), become more prominent in chronic stage.3,6,8–10 The findings are supported by postmortem studies that showed degeneration, loss of axon integrity, demyelination, and microglial infiltration but the causes of this damage to cerebral white matter in schizophrenia remain unknown. The postmortem studies in patients provide evidence for infiltration of microglia, reactive macrophages, and astrogliosis were thought to be involved in decreasing of white matter integrity.5 In parallel, patients with schizophrenia are characterized by abnormal cytokine levels that can cross blood-brain barrier from peripheral to central nervous system and activating microglia.11 Elevated of certain cytokines from the periphery or centrally activated microglia are known to affect oligodendrocyte precursor cells.4,11–14
Activated immune-inflammatory response system (IRS), represented by proinflammatory cytokines, and mild immunosuppression with the compensatory immune-regulatory reflex system (CIRS), represented by anti-inflammatory cytokines, were the characteristics of peripheral blood in patients with schizophrenia.15,16 We have previously organized the extensive battery of cytokines into IRS and CIRS.17 IRS/CIRS ratio indicates the disequilibrium of proinflammatory and anti-inflammatory cytokines.18 A meta-analysis including 10 studies with antipsychotic naive first-episode schizophrenia showed that IRS was significantly activated in the schizophrenia group with medium–large effect size of IFN-γ, IL6, IL12, and IL17, compared with the healthy control group. The results also pointed out that IL-1β, IL2, IL6, and TNF-α were positively correlated with severity of negative symptoms, while the anti-inflammatory cytokine IL10 was negatively correlated with negative symptoms score, and IL4 was positively correlated with strong effect size.19 Associated with decreased brain-derived neurotrophic factor, IRS/CIRS could exert neurotoxic effect in the development of first-episode schizophrenia.20 Positively associated with enlarged lateral ventricle volume and psychiatric symptom severity, IRS/CIRS disequilibrium was also observed in patients with treatment-resistant schizophrenia.18 To the best of our knowledge, few studies focused on the underlying mechanism of relationship between IRS/CIRS and severity of negative symptoms.
We found that the increased IRS/CIRS ratio, representing activated IRS, and/or insufficient CIRS, was negatively associated with whole-brain white matter average fractional anisotropy (FA) in FEPS.21 Proinflammatory cytokines could exerts an inhibitory role in the survival and differentiation or recruitment of oligodendrocyte progenitor cells to inhibit white matter restoration and functional renewal.22,23 Proinflammatory cytokines could also prevent the development of oligodendrocytes by hindering the expression of myelin basic protein mRNA24 or inducing demyelination.25 Some of these effects can be regionally specific, for example, increased level of plasma IL6 was associated with reduced the FA values of genu of corpus callosum (GCC) and uncinate fasciculus, speculated to be related to glial cell dysfunction, myelin damage, and/or axonal impairment.8,14 Importantly, these regions are among the most implicated white matter tracts in patients with schizophrenia.8,14 Collectively, these studies shadowed that peripheral inflammation may affect white matter integrity.
Here, we proposed to use the composite IRS/CIRS score to evaluate the relationship between integrity of white matter and clinical symptom severity in patients with schizophrenia. Specifically, we aimed to show that (1) plasma IRS/CIRS is significantly higher in patients with schizophrenia (SCZ) group than healthy controls (HCs), (2) elevated IRS/CIRS is inversely associated with white matter integrity, and (3) the severity of clinical symptoms was associated with IRS/CIRS by decreasing the integrity of specific white matter tracts as measured by their mediation effects.
Materials and Methods
Participants
We recruited 120 patients with SCZ in Beijing Huilongguan Hospital and 65 age and sex-frequency-matched HCs in local communities using advertisements. All patients in SCZ group met the following criteria: (1) diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV), (2) 16–65 years old, and (3) at least 6 years of education. The medication status of patients was shown as following, risperidone (32), olanzapine (26), clozapine (18), aripiprazole (13), haloperidol combined with either risperidone or olanzapine (8), drug naïve (6), paliperidone (5), aripiprazole combined with risperidone or olanzapine or clozapine (3), haloperidol (2), quetiapine (2), perphenazine (2), quetiapine combined with risperidone (1), sulpiride (1), lurasidone (1). Daily antipsychotic dosage was converted to chlorpromazine equivalents. The exclusion criteria were: (1) other psychiatric disorders diagnosed according to DSM-IV Axis I diagnosis, (2) serious physical illness, (3) recent infectious diseases or treated with immunotherapy, physiotherapy or psychotherapy, (4) mental retardation or severe neurological disease, (5) lactation or pregnancy. HCs group has not diagnosed psychiatric disorders according to DSM-IV Axis I in the participants and their first-degree relatives. table 1 showed the demographic data of SCZ and HCs groups. All participants of this study had signed written informed consent before any experiment performance was done. The study protocol was approved by the Ethics Committee of the Beijing Huilongguan Hospital. Study procedures met the ethical standards of the Declaration of Helsinki. The privacy rights of all subjects were always observed. The white matter integrity value and cytokines from 42 SCZ and 42 HCs were reported in the previous article.21
Table 1.
Demographics and Clinical Characteristics of SCZ and HCs
| Characteristics | SCZ(n = 120) Mean (SD) |
HCs(n = 65) Mean (SD) |
Statistics (χ2/t) |
P-value |
|---|---|---|---|---|
| Sex (M/F) | 70/50 | 38/27 | 2.85 × 10−4 | .987 |
| Age (years) | 40.76 (13.10) | 37.48 (12.31) | 1.66 | .098 |
| Education (year) | 12.23(3.09) | 13.42(2.54) | −2.66 | .009 |
| BMI (kg/m2) | 24.08(4.39) | 24.03(3.11) | 0.86 | .932 |
| Illness duration (month) | 19.99(13.04) | NA | NA | NA |
| PANSS | ||||
| Total Score | 69.49(19.45) | NA | NA | NA |
| P-Subscale | 18.62(7.35) | NA | NA | NA |
| N-Subscale | 17.58(7.34) | NA | NA | NA |
| G-Subscale | 33.30(9.73) | NA | NA | NA |
| CPZ | 346.91(214.12) | NA | NA | NA |
Note: SCZ, patients with schizophrenia; HCs, healthy controls; BMI, body mass index; PANSS, Positive and Negative Symptom Scale; CPZ, antipsychotic dosage by chlorpromazine equivalents; NA, not application.
The bold font represents P < .05.
Peripheral Cytokine Measurement
EDTA-K2 disposable vacuum collection tubes (Beijing Dongfang Jianfeng Technology Co. Ltd) were used to collect plasma of SCZ and HCs on the same day subjects were interviewed by psychiatrists. The plasma samples were centrifuged and then aliquoted and stored at −80°C. Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) kits (Beijing Rongxin Zhihe Biotechnology Co. Ltd., China) were used to measure the concentration of these 16 plasma cytokines markers—IFNγ, IL1β, IL2, IL6, IL8, IL12, IL17, IL18, TNFα, IL1Ra, IL4, IL10, sIL2Ra, sIL2Rb, TGFβ1, and TGFβ2. The procedures were done according to the manufacturer’s instruction26 and the experimenters were blind to group assignment. In brief, the procedures were to set the standard, sample, and blank wells; add different concentrations of standards, diluted samples to be tested and nothing to these wells, respectively; add HRP-Conjugate reagent to all the wells except the blank wells, and incubate at 37°C for 30 minutes; aspirate and wash the wells and add chromogen A and B and incubate at 37°C for 15 minutes. Next, stop buffer was added to wells; the O.D. (optical density) was detected using 800TMTS Absorbance Reader (BioTek instrument, America). The intra- and inter-assay variation coefficients were less than 10% and 15%, respectively.
Measurement of Symptom Severity
Two psychiatrists independently interviewed all participants enrolled in this study. Diagnosis was made using DSM-IV. The psychiatric symptom severity of patients was assessed by Positive and Negative Syndrome Scale (PANSS). The interviewers maintained an intra-class correlation coefficient (ICC) above 0.75 on these instruments.
Imaging and Data Processing
A 3-T Prisma MRI scanner (Siemens Medical Solutions, Germany) with a 64-channel head coil was used to acquire the structural MRI and DTI image data in Beijing Huilongguan Hospital imaging research center. All participants who completed the MRI scanning did not have MRI contraindications. Padding was used to retrain and minimize the movement of patients during scanning. We collected structural T1-weighted and DTI imaging data following the established parameters27–30 and extracted data on volumes of subcortical gray matter structures using T1 and white matter microstructural data using DTI. In brief, sagittal 3D magnetization prepared rapid acquisition gradient echo (MP-RAGE) was used to acquire structural T1-weighted images and the scan parameters were as follows: Echo time (TE) = 2.98 ms, repetition time = 2 530 ms, flip angle (FA) = 7°, matrix size = 256 × 224, field-of-view = 256 × 224 mm, inversion time (TI) = 1 100 ms, and thickness/gap = 1/0 mm. FreeSurfer (version 5.3) was used to perform subcortical structural volumetric processing. A standard procedure, including motion correction, nonbrain tissue removal, automated Talairach transformation, and subcortical volumetric structures segmentation, was used to extract volumetric measurements.31,32 We included lateral ventricular, choroid plex, thalamus, amygdala, hippocampus, caudate, putamen, pallidum, accumbens into analysis, and summing left and right volumes of the same structures.28,29 We employed ENIGMA-DTI analysis pipeline to process DTI data.33 All data passed ENIGMA-DTI quality assurance and control. Based on ENIGMA-DTI atlas, 21 major regional white matter FA were generated and averaged across hemispheres.
Statistical Analysis
SPSS 20.0 and GraphPad Prism 8.0 were used to make statistical analyses and graphs, respectively. Categorical variables, such as sex, were compared using χ2 test. Shapiro–Wilk test were used to test the normality of all continuous data. Continuous variables with normal distribution, such as age, education years, body mass index (BMI), some cytokines, and white matter average FA were compared between groups using unpaired t-test and other non-normally distributed continuous variables using Mann–Whitney U-test. Cohen’s d, the effect size of difference between schizophrenia group and HCs group in kinds of cytokine and 3 types of cytokine profiles, was calculated using JASP 0.16.1(JASP Team, Amsterdam, The Netherlands). To evaluate relationships between biological indicators and FA of white matter or subcortical structures, we used partial correction analysis, with age, sex, years of education, BMI, and total intracranial volume as covariates.11,17 Significance level was set at α < 0.05. Bonferroni correction was applied in multiple comparisons.
z unit weighted composite score15,34,35 for cytokines profiles was calculated as below and described in table 2.
Table 2.
Cytokine Levels and Different Types of Cytokine Profiles Expressed in Plasma of SCZ and HCs.
| SCZ(n = 120) Mean (SD) |
HCs(n = 65) Mean (SD) |
Statistics (F/t) |
P-value | |
|---|---|---|---|---|
| IRS | 1.44(8.36) | 0.00(7.40) | 1.16 | .248 |
| CIRS | −0.14(5.94) | 0.00(5.73) | −0.15 | .878 |
| IRS/CIRS | 1.57(4.42) | 0.00(3.55) | 2.64 | .009 |
| IFNγ (pg/ml) | 913.14(369.48) | 800.02(279.90) | 2.16 | .032 |
| IL1β (pg/ml) | 106.09(42.7) | 99.05(45.69) | 1.04 | .298 |
| IL2 (ng/ml) | 9.89(3.16) | 9.66(3.08) | 0.47 | .636 |
| IL6 (pg/ml) | 54.92(19.29) | 50.47(18.34) | 1.52 | .130 |
| IL8 (pg/ml) | 125.72(50.89) | 119.24(36.42) | 0.91 | .366 |
| IL12 (pg/ml) | 40.35(14.26) | 43.16(14.34) | −1.28 | .202 |
| IL17 (pg/ml) | 324.58(116.86) | 328.26(144.25) | −0.19 | .851 |
| IL18 (pg/ml) | 356.99(119.6) | 339.07(89.06) | 1.15 | .250 |
| TNFα(pg/ml) | 88.07(26.28) | 78.90(22.69) | 2.37 | .019 |
| IL1Ra (pg/ml) | 203.82(83.73) | 199.90(63.58) | 0.33 | .742 |
| IL4 (pg/ml) | 47.87(16.45) | 53.71(17.62) | −2.25 | .026 |
| IL10 (pg/ml) | 840.98(237.8) | 919.23(406.21) | −1.43 | .157 |
| sIL2Ra (pg/ml) | 404.26(121.38) | 390.01(107.34) | 0.79 | .429 |
| sIL2Rb (pg/ml) | 5173.98(1830.21) | 4925.39(2185.81) | 0.78 | .437 |
| TGFβ1(ng/ml) | 254.05(124.47) | 242.64(102.75) | 0.63 | .529 |
| TGFβ2(pg/ml) | 3605.00(1045.98) | 3645.87(1203.33) | −0.24 | .810 |
Note: SCZ, patients with schizophrenia; HCs, healthy controls. IRS, immune-inflammatory response system, computed as z composite score of IFNγ, IL1β, IL2, IL6, IL8, IL12, IL17, IL18, and TNFα.
CIRS, compensatory immune-regulatory system, computed as z composite score of IL1Ra, IL4, IL10, sIL2Ra, sIL2Rb, TGFβ1, and TGFβ2. IRS/CIRS, computed as z(IRS) – z(CIRS).
The bold font represents P < .05.
z = (actual value of the variable–mean of the variable in the healthy sample)/standard deviation of the variable in the healthy sample36
IRS: Immune IRS, computed as z value of IFNγ (zIFNγ) + zIL1β + zIL2 + zIL6 + zIL8 + zIL12 + zIL17 + zIL18 + zTNFα.
CIRS: Compensatory immune-regulatory system, computed as zIL1Ra + zIL4 + zIL10 + zsIL2Ra + zsIL2Rb + zTGFβ1 + zTGFβ2.
IRS/CIRS: Computed as z(IRS) – z(CIRS).
PROCESS v3.5 was used to perform the mediation analysis in SPSS. The 95% confidence intervals of indirect and direct effects were obtained with 5000 bootstrap samples. The regression coefficient (β) in each path indicated the magnitude and direction. If the confidence interval did not contain zero, the indirect effect was significant.
Results
Demographics and Clinical Characteristics
One hundred and twenty SCZ and 65 HCs completed both cytokine measurements and DTI. There was no significant difference in sex ratio, age, and BMI between SCZ and HCs. SCZ group acquired fewer years of education (both P < .001), compared to HCs (table 1). The PANSS total score and its 3 subscale scores, and antipsychotic dosage by chlorpromazine equivalents (CPZ) are also shown in table 1.
Group Differences in Peripheral Cytokines
The SCZ group had elevated IFNγ, TNFα, and reduced IL4 expression levels (all P < .05; table 2). The IRS/CIRS ratio score in SCZ group were significantly higher than that in HCs (P = .009); and IRS and CIRS were not (P = .248, P = .878, respectively) (figure 1A). The effect sizes of the composite scores and the individual cytokines were displayed in figure 1B. The measure with the largest effect size was IRS/CIRS ratio (d = 0.39).
Fig. 1.
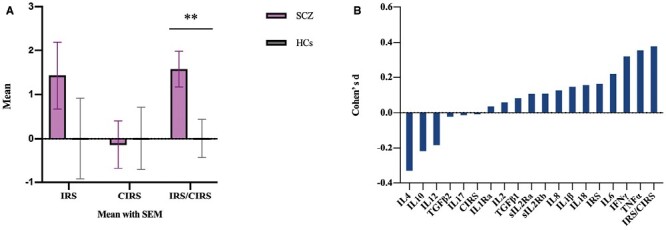
Group and effect sizes differences in SCZ and HCs. (A) Group differences in peripheral cytokines in SCZ and HCs. (B) Effect sizes of difference between SCZ group and HCs group in kinds of cytokine and 3 types of cytokine profiles. The largest effect size was in inflammatory response system (IRS)/CIRS (d = 0.393). SCZ, patients with schizophrenia; HCs, healthy controls. ** Significant at P < .01.
IRS/CIRS Ratio and White Matter Average FA
SCZ had significantly lower whole-brain white matter average FA compared to HCs, (t = −5.44, P < .001) (figure 2A). Whole-brain white matter average FA of SCZ and HCs were both correlated with IRS/CIRS in a negative trend, but not significant (both P > .05, after adjusting for age, sex, education, BMI, and total intracranial volume) (figure 2B).
Fig. 2.
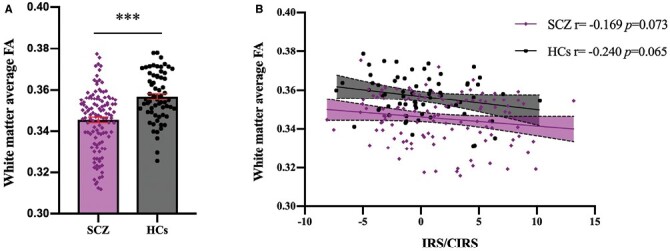
Relationship between inflammatory response system (IRS)/CIRS and whole-brain white matter in SCZ and HCs. The average fractional anisotropy of SCZ was significantly lower than that of HCs (t = −5.44, P < .001) (A) and both groups were associated with IRS/CIRS in a negative trend, but not significant, taking age, sex, education, body mass index, and total intracranial volume as covariates. (B). SCZ, patients with schizophrenia; HCs, healthy controls. *** Significant at P < .001.
Specific White Matter Region and IRS/CIRS Ratio
Among the 21 white matter microstructural regions, the FA of 15 were significantly lower in SCZ, compared to HCs, displayed in figure 3A. Of these 15 regions, 10 of them were nominally significantly associated with IRS/CIRS ratio (all p < 0.05), with the GCC remaining significant after Bonferroni correction of multiple comparisons (figure 3B). Taking age, sex, education, BMI, and total intracranial volume as covariates, the partial correlation of IRS/CIRS ratio and the FA of GCC was significant in SCZ (r = −0.29, P = .002). This was not significant but in the same direction in the HCs (r = −4.02 × 10−4, P = .10) (figure 3C).
Fig. 3.
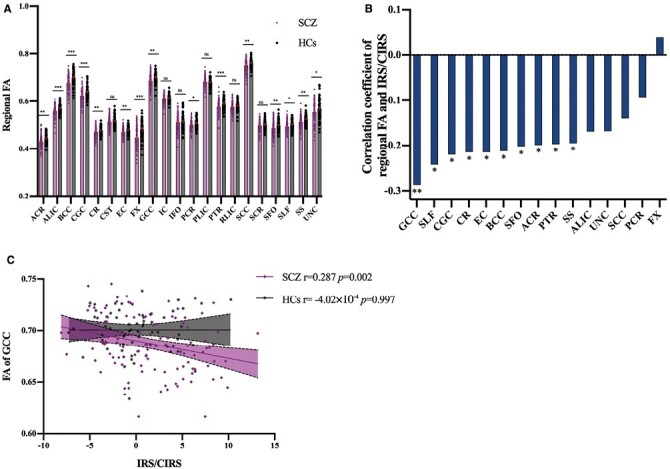
Correlation of regional fractional anisotropy (FA) and inflammatory response system (IRS)/CIRS. FA of 15 white matter microstructural regions were significantly lower in SCZ (A). 10 of them were nominally significantly associated with IRS/CIRS ratio, regarding age, sex, education, body mass index, and total intracranial volume as covariates. (all P < .05) (B). Only FA of GCC and IRS/CIRS was significantly negatively correlated in schizophrenia patients (P= .002, significant after FDR), but not in HCs (P > .05) (C) SCZ, patients with schizophrenia; HCs, healthy controls; ACR, anterior corona radiata; ALIC, anterior limb of internal capsule; BCC, body of corpus callosum; CGC, cingulum; CR, corona radiata; CST, cortico-spinal tract; EC, external capsule; FX, fornix; GCC, genu of corpus callosum; IC, internal capsule; IFO, inferior frontal occipital fasciculus; PCR, posterior corona radiata; PLIC, posterior limb of internal capsule; PTR, posterior thalamic radiation; RLIC, retrolenticular limb of the internal capsule; SCC, splenium of corpus callosum; SCR, superior corona radiata; SFO, superior fronto-occipital fasciculus; SLF, superior longitudinal fasciculus; UNC, uncinate fasciculus; SS, sagittal striatum. * Significant at P < .05; ** Significant at P < .01; *** Significant at P < .001; ns, not significant.
FA of GCC, IRS/CIRS, PANSS Score, Illness Duration, and CPZ
Considering age, sex, education, BMI, and total intracranial volume as covariates, there was no correlation between FA of GCC and illness duration or CPZ (both partial P > .05). IRS/CIRS ratio was also not associated with illness duration or CPZ (both P > .05). FA of GCC was negatively correlated with PANSS negative subscale score (r = −0.23, P = .016) (figure 4). PANSS negative subscale score was also not associated with illness duration or CPZ (both partial P > .05). IRS/CIRS was not associated with PANSS subscale scores and total score (P > .05) and there was no mediation effect taking FA of GCC as the mediator.
Fig. 4.
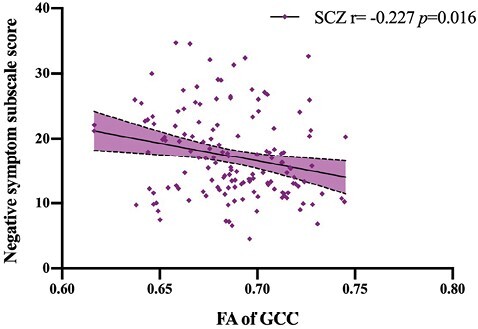
Correlation of fractional anisotropy (FA) of genu of corpus callosum (GCC) and Positive and Negative Syndrome Scale (PANSS) score. The FA of GCC was negatively associated with PANSS negative subscale score in SCZ, considering age, sex, education, body mass index, and total intracranial volume as covariates. (P = .016).
IRS/CIRS and Volume of Subcortical Structures
Among the 9 subcortical substructures, lateral ventricle was significantly increased in SCZ and thalamus, amygdala, hippocampus, accumbens, and choroid plexus were significantly reduced, compared to HCs (table 3). These 6 structures were not associated with IRS/CIRS ratio. The volume of caudate, which was insignificantly different between SCZ and HC, showed significantly positively associated with IRS/CIRS (r = 0.24, P = .007), although that was not significant after Bonferroni correction for multiple comparisons (figure 5).
Table 3.
comparisons in volume of subcortical structures between SCZ and HCs.
| Subcortical Structures | SCZ(n = 120) Mean (S.D.) |
HCs(n = 65) Mean (S.D.) |
Statistics (t) |
P-value |
|---|---|---|---|---|
| Lateral ventricle | 19.93(10.65) | 14.02(6.2) | 4.754 | <.001 |
| Thalamus | 14.79(1.54) | 15.62(1.85) | −3.214 | .002 |
| Caudate | 6.90(0.93) | 6.99(0.90) | −0.634 | .527 |
| Putamen | 10.14(1.23) | 10.33(1.28) | −0.940 | .348 |
| Pallidum | 4.22(0.46) | 4.18(0.44) | 0.619 | .537 |
| Amygdala | 7.79(0.80) | 8.24(0.69) | −3.799 | <.001 |
| Hippocampus | 3.29(0.38) | 3.46(0.4) | −2.828 | .005 |
| Accumbens | 0.89(0.17) | 0.97(0.17) | −2.886 | .004 |
| Choroid plexus | 1.12(0.42) | 0.88(0.35) | 3.913 | <.001 |
Note: SCZ, patients with schizophrenia; HCs, healthy controls.
The bold font represents P < .05.
Fig. 5.
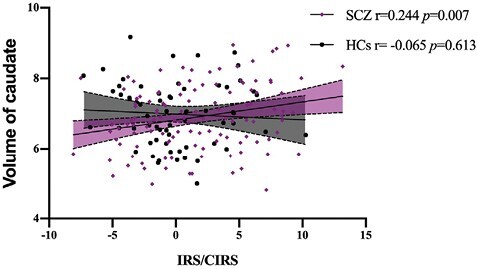
Correlation between inflammatory response system (IRS)/CIRS and volume of caudate. The volume of caudate in SCZ was significantly positively associated with IRS/CIRS. The result remained significant after including age, sex, education, body mass index, and total intracranial volume as covariates.
Discussion
In this study, we found that the IRS/CIRS ratio was significantly higher in patients with schizophrenia than that in HCs and the average white matter FA was significantly decreased in the patient group. IRS/CIRS ratio was associated with whole-brain white matter average FA in both groups in a negative trend. Of the 21 white matter microstructural fiber tracts, the FA value of 15 tracts was significantly reduced in SCZ compared to controls. Only the FA value of GCC was significantly associated with IRS/CIRS ratio after Bonferroni correction. IRS/CIRS ratio was insignificantly associated with negative symptoms in SCZ. FA of GCC was negatively associated with negative symptoms in SCZ. None of the subcortical substructures were significantly associated with IRS/CIRS ratio after correction for multiple comparisons.
Our first hypothesis was that plasma IRS/CIRS is higher in SCZ group. Our result was in line with the finding of Maes in patients with schizophrenia, both first-episode and chronic.15,20,37 It seems that the robust IRS, activated but insufficient CIRS and finally elevated IRS/CIRS are trait makers of schizophrenia. IFNγ and TNFα were the representative cytokines of IRS and significantly higher in schizophrenia, consistent with the meta-analysis showed that TNFα and IFNγ were significantly increased in the phase of first-episode schizophrenia, acute exacerbation of chronic schizophrenia, treatment of acute psychosis, and chronically illness patients, except for IFNγ levels significantly decreased in chronically patients.17 IL1β and IL6 were reported increased in FEPS and decreased after treatment17 and they showed no difference with controls in our sample. Compared to FEPS, some of the patients enrolled in our study were in chronic stage and the CIRS was slightly lower than that in FEPS and HC (supplementary figure S1 and figure 1A). The IRS was also slightly activated, but not that much in our present study, compared to FEPS. It seems that in chronic patients the anti-inflammatory system and proinflammatory system are not effectively activated with low-grade inflammation and smaller effective size than FEPS compared to HCs due to some reasons, such as antipsychotic treatment and chronic stress leading to decreased glucocorticoid receptor sensitivity with hypothalamic-pituitary-adrenal axis deficits.17,37 This phenotype is similar in elder people and some researchers define it as inflammageing, aging of the immune system, with persistent and unsolved production of low-level pro-inflammatory mediators38,39 and loss of homeostasis.
Causing by “continuous antigenic and stress,” immunosenescence also exists in psychosis.40 Patients with schizophrenia were reported to suffer from infection and psychosocial stress before onset and continued for a live long time, such as prenatal infection, trauma in childhood, cytomegalovirus infection, toxoplasma infection, social, and economic stress.41–43 These stressors and chronic antigen load promote the continuous consumption and remodeling of the immune system39 and eventually premature immunosenescence. This may give rise to significant proinflammatory signaling and explain the reason of low-level proinflammation in patients with schizophrenia. Triggered by low-grade proinflammatory, the anti-inflammatory, for example, IL4, in patients with schizophrenia was significantly decreased in our study. In some treatment-resistant schizophrenia and drug-naive adolescents with first episode, early onset schizophrenia, anti-inflammatory cytokines, IL4, and IL10, were found no significant difference with HCs44,45 or decreased in the meta-analysis results of schizophrenia blood cytokine network alterations.17 As a result, CIRS was not sufficiently activated and its composite score was also lower than HCs. The imbalance of pro- and anti-inflammatory may have detrimental consequences and develop inflammatory disease,46 causing some changes in tissue microstructures, like white matter.
Our second hypothesis was that elevated IRS/CIRS is inversely associated with average FA. Peripheral cytokines could pass through blood-brain barrier by saturable transporter for TNFs, ILs and IFN, and promote the migration of CD4 + T cells and monocytes across blood-brain barrier.4,47,48 The result of a recent study also showed that TNFα highly expressed in brain endothelial cells and microglial cells in schizophrenia postmortem individuals and the KEGG pathway analysis showed the genes related to TNF signaling were enriched.49 Interact with these immune cells and cytokines, activated microglia release inflammatory mediators and accumulate around some specific brain regions, including hippocampus, corpus callosum, caudate putamen, and dentate gyrus,50–52 all of which are regions strongly implicated in schizophrenia. Along with increased inflammatory cytokines in white matter, the number of myelinated axons with reduced diameter and nonmyelinated axons are increased, and developing oligodendrocytes decreased.53 In conclusion, in patients with schizophrenia, excessive peripheral or microglial cytokines exert influence on the maturation of oligodendrocyte progenitor cells and myelination of oligodendrocyte, and promote demyelination, and finally reduce the integrity of white matter. However, with the development of the disorder and immunosenescence, in chronic patients with schizophrenia, IRS/CIRS is unlikely to be the sole driver of white matter integrity decreasing. NMDA receptor antibody,28 carbonyl stress and pyridoxal,54 glucose metabolism,55 genetic variants,56 and DNA methylation57 were also reported to be associated with the deficit integrity of white matter.
During the partial correction analysis of the relationship between IRS/CIRS and white matter microstructural fiber tracts, we found that the integrity of most white matter fasciculus were decreased in SCZ and only the FA of GCC was significantly inversely associated with IRS/CIRS after Bonferroni correction, taking age, sex, education, BMI, and total intracranial volume as covariates. Functionally connecting left and right frontal and cingulate cortex, GCC undergirds affective, and cognitive processing.1,4 It is reported to be vulnerable to inflammatory signaling, for CC is known to be the brain area with the greatest number of microglia and oligodendrocyte.58 Increased proinflammatory cytokines, like TNFα and IL1β, would damage axon and the white matter essential component—myelin sheath, through demyelination, oligodendrocyte, or inhibition of progenitor cells maturation.53,58,59 Moreover, quetiapine blocked the effect of cuprizone on neuroinflammation—elevated proinflammation, and oligodendrocyte loss in the mouse model.60 Perhaps, ameliorating the neuroinflammation of GCC is beneficial for improving its functional connectivity among frontal and cingulate cortex.
The third hypothesis was that the severity of clinical symptom clusters was associated with IRS/CIRS by decreasing the integrity of specific white matter tracts. After adjusting age, sex, education, BMI, and total intracranial volume, we found that FA of GCC was negatively associated with negative subscale score in SCZ, in line with the results reported by Nakamura et al6 Taking age, sex, education, and drug usage as covariates, Abdolalizadeh et al2 also found that apathy-avolition was associated with the severity of dysconnectivity in white matter and showed the central role of the corpus callosum in apathy. They also pointed out that inflammation in peripheral and neuroinflammation were more related to negative and cognitive symptoms.2 However, we did not find the association between negative symptoms and IRS/CIRS in our study, due to our relatively small size and heterogeneity of sample. GCC is related to reward networks and diminished emotional expression, which may underlie the association with negative symptoms.1 some studies also found that positive symptom is associated with GCC,1,61 because of the heterogeneity of illness or methodological differences.6 Minocycline, which has an anti-inflammatory effectiveness, are demonstrated to strengthen the role of antipsychotics in the treatment of negative symptoms of schizophrenia.62,63 More studies are needed to clarify the relationship among IRS/CIRS, white matter, and negative symptoms in schizophrenia.
Some proinflammatory cytokines could decrease neurogenesis in hippocampus, amygdala, caudate, body, and splenium of the corpus callosum o and cause anatomical volume changes, which correlated with severity of symptoms in schizophrenia.18,64–66 To explore the effect IRS/CIRS exert on other brain structures except for white matter, which was less studied, we found that the volume of caudate was positively associated with IRS/CIRS in SCZ, in spite of the not significant difference in caudate volume between the 2 groups. Caudate was a not-well-studied subcortical brain structure in schizophrenia with neuroinflammation. Under systemic inflammation, the activity of DA remission to a stressor was enhanced in caudate.67 Quetiapine and N-acetylcysteine were reported to ameliorate the microglia activation in caudate area through its anti-inflammatory actions.52,60 These phenomena could account for the role of IRS/CIRS in the development of schizophrenia symptoms and treatment of antipsychotics, at least partly.
There are some limitations in our study. Firstly, our study was a cross-sectional study, hindering our exploration of the casual relationship between IRS/CIRS and white matter tracts and subcortical brain structures. Secondly, the range of age in our sample was large, from 16 to 65 years old. Aging has a certain effect on white matter. However, one research pointed out that the effects of cytokines on white matter integrity reduction were larger in elder ages compared to younger ages (<67 years).68 We also take age as covariate to minimize its effect on white matter. Thirdly, illness duration and medicine might be biases for our findings. However, one-way ANOVA analyses showed that the heterogeneity of disease duration and medication effects might not associate with disease progression (FA of GCC and severity of negative symptom), displayed in supplementary tables S1–S3.
In conclusion, peripheral IRS/CIRS was significantly elevated in patients with schizophrenia, which might act as a trite marker for schizophrenia. IRS/CIRS could partly account for the severity of negative symptoms by targeting the integrity of GCC. Anti-inflammatory therapy is a very promising treatment option for schizophrenia by ameliorating the imbalance of IRS and CIRS.
Supplementary Material
Acknowledgments
We would like to thank all of the study participants and staff. Yunlong Tan, Li Tian and L. Elliot Hong designed the project and obtained the funding for this study. Mengzhuang Gou, Wenjin Chen, Yanli Li, Song Chen, Wei Feng, Shujuan Pan, Wei Li, Jinghui Tong, Yanfang Zhou, Hongna Li, Ting Yu, Ping Zhang, and Junchao Huang were responsible for recruiting patients, performing clinical ratings, neuroimaging, and collecting samples. Mengzhuang Gou analyzed all the data and wrote the article. Yunlong Tan and Li Tian are responsible for the integrity of data and the accuracy of data analysis. Baopeng Tian, Zhiren Wang, Shuping Tan, Xingguang Luo, Chiang-Shan R. Li, Peter Kochunov, and L. Elliot Hong were invited in evolving the ideas and editing the manuscript. All authors have contributed to and have approved the final manuscript. LEH has received or plans to receive research funding or consulting fees on research projects from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Luye Pharma, Sound Pharma, IGC Pharma, Takeda, and Regeneron. None was involved in the design, analysis, or outcomes of the study. All other authors declare no competing commercial and financial interests.
Contributor Information
Mengzhuang Gou, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Wenjin Chen, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Yanli Li, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Song Chen, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Wei Feng, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Shujuan Pan, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Xingguang Luo, Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
Shuping Tan, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Baopeng Tian, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Wei Li, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Jinghui Tong, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Yanfang Zhou, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Hongna Li, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Ting Yu, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Zhiren Wang, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Ping Zhang, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Junchao Huang, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Peter Kochunov, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD, USA.
Li Tian, Institute of Biomedicine and Translational Medicine, Department of Physiology, Faculty of Medicine, University of Tartu, Tartu, Estonia.
Chiang-Shan R Li, Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
L Elliot Hong, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD, USA.
Yunlong Tan, Peking University HuiLongGuan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, China.
Funding
This work was supported by the capital health research and development of special (2022-1-2131), the National Natural Science Foundation of China grant (82171507, 81761128021, and 81771452), the National Institute of Health grants (R01MH112180), the Estonian Research Council-European Union Regional Developmental Fund Mobilitas Plus Program No. MOBTT77 and the Estonian Research Council personal research funding team grant project No. PRG878, the National Natural Science Foundation of China (82001415), the Capital’s Funds for Health Improvement and Research (CFH2020-2-2134). The funding source had no involvement in the study design, in the collection, analysis, and interpretation of data, in the writing of the article, and in the decision to submit the article for publication.
References
- 1. Lim J, Chin R, Ho NF, et al. Elucidation of shared and specific white matter findings underlying psychopathology clusters in schizophrenia. Asian J Psychiatr. 2017;30:144–151. [DOI] [PubMed] [Google Scholar]
- 2. Abdolalizadeh A, Ostadrahimi H, Ohadi MAD, Saneei SA, Bayani Ershadi AS.. White matter microstructural associates of apathy-avolition in schizophrenia. J Psychiatr Res. 2021;142:110–116. [DOI] [PubMed] [Google Scholar]
- 3. Cho SJ, Kim MK, Bang SY, Bang M, Lee S-H.. White matter integrity associated with severity reductions in positive symptoms after amisulpride treatment in drug-free patients with schizophrenia. Neurosci Lett. 2018;685:131–136. [DOI] [PubMed] [Google Scholar]
- 4. Ho TC, Kulla A, Teresi GI, et al. Inflammatory cytokines and callosal white matter microstructure in adolescents. Brain Behav Immun. 2022;100:321–331. [DOI] [PubMed] [Google Scholar]
- 5. Cardenas AM, Sarlls JE, Kwan JY, et al. Pathology of callosal damage in ALS: an ex-vivo, 7 T diffusion tensor MRI study. Neuroimage Clin. 2017;15:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamura K, Kawasaki Y, Takahashi T, et al. Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: a voxel-based diffusion tensor imaging study. Psychiatry Res. 2012;202(3):233–238. [DOI] [PubMed] [Google Scholar]
- 7. Ellison-Wright I, Bullmore E.. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Wei Y, Edmiston EK, et al. Altered structural connectivity and cytokine levels in Schizophrenia and Genetic high-risk individuals: associations with disease states and vulnerability. Schizophr Res. 2020;223:158–165. [DOI] [PubMed] [Google Scholar]
- 9. Reis Marques T, Taylor H, Chaddock C, et al. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain. 2014;137(Pt 1):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saito J, Hori M, Nemoto T, et al. Longitudinal study examining abnormal white matter integrity using a tract-specific analysis in individuals with a high risk for psychosis. Psychiatry Clin Neurosci. 2017;71(8):530–541. [DOI] [PubMed] [Google Scholar]
- 11. Michalczyk A, Tyburski E, Podwalski P, et al. Serum inflammatory markers and their associations with white matter integrity of the corpus callosum in schizophrenia patients and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2022;116:110510. [DOI] [PubMed] [Google Scholar]
- 12. Malashenkova IK, Krynskiy SA, Ogurtsov DP, et al. A role of the immune system in the pathogenesis of schizophrenia. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118(12):72–80. [DOI] [PubMed] [Google Scholar]
- 13. Malashenkova IK, Krynskiy SA, Ogurtsov DP, et al. A role of the immune system in the pathogenesis of schizophrenia. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118(12):72–80. [DOI] [PubMed] [Google Scholar]
- 14. Najjar S, Pearlman DM.. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102–112. [DOI] [PubMed] [Google Scholar]
- 15. Noto MN, Maes M, Nunes SOV, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29(3):416–431. [DOI] [PubMed] [Google Scholar]
- 16. Maes M, Berk M, Goehler L, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldsmith DR, Rapaport MH, Miller BJ.. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen W, Gou M, Wang L, et al. Inflammatory disequilibrium and lateral ventricular enlargement in treatment-resistant schizophrenia. Eur Neuropsychopharmacol. 2023;72:18–29. [DOI] [PubMed] [Google Scholar]
- 19. Dunleavy C, Elsworthy RJ, Upthegrove R, Wood SJ, Aldred S.. Inflammation in first-episode psychosis: the contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;146(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noto MN, Maes M, Vargas Nunes SO, et al. BDNF in antipsychotic naive first episode psychosis: effects of risperidone and the immune-inflammatory response system. J Psychiatr Res. 2021;141:206–213. [DOI] [PubMed] [Google Scholar]
- 21. Gou M, Li W, Tong J, et al. Correlation of immune-inflammatory response system (irs)/ compensatory immune-regulatory reflex system (CIRS) with white matter integrity in first-episode patients with schizophrenia. Mol Neurobiol. 2023. (under review). [DOI] [PubMed] [Google Scholar]
- 22. Benedetti F, Poletti S, Hoogenboezem TA, et al. Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. J Affect Disord. 2016;202:1–9. [DOI] [PubMed] [Google Scholar]
- 23. Zhou Y, Zhang J, Wang L, et al. Interleukin-1beta impedes oligodendrocyte progenitor cell recruitment and white matter repair following chronic cerebral hypoperfusion. Brain Behav Immun. 2017;60:93–105. [DOI] [PubMed] [Google Scholar]
- 24. Seki Y, Kato TA, Monji A, et al. Pretreatment of aripiprazole and minocycline, but not haloperidol, suppresses oligodendrocyte damage from interferon-γ-stimulated microglia in co-culture model. Schizophr Res. 2013;151(1–3):20–28. [DOI] [PubMed] [Google Scholar]
- 25. Prasad KM, Upton CH, Nimgaonkar VL, Keshavan MS.. Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: an integrated DTI study. Schizophr Res. 2015;161(1):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gou M, Pan S, Tong J, et al. Effects of microRNA-181b-5p. on cognitive deficits in first-episode patients with schizophrenia: Mediated by BCL-2. J Psychiatr Res. 2021;136:358–365. [DOI] [PubMed] [Google Scholar]
- 27. Kochunov P, Huang J, Chen S, et al. White matter in schizophrenia treatment resistance. Am J Psychiatry. 2019;176(10):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tong J, Zhou Y, Huang J, et al. N-methyl-D-aspartate receptor antibody and white matter deficits in schizophrenia treatment-resistance. Schizophr Bull. 2021;47(5):1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou YF, Huang JC, Zhang P, et al. Choroid plexus enlargement and allostatic load in schizophrenia. Schizophr Bull. 2020;46(3):722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou Y, Huang J, Zhang P, et al. Allostatic load effects on cortical and cognitive deficits in essentially normotensive, normoweight patients with schizophrenia. Schizophr Bull. 2021;47(4):1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. [DOI] [PubMed] [Google Scholar]
- 32. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation- automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 33. Jahanshad N, Kochunov PV, Sprooten E, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maes M, Carvalho AF.. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol. 2018;55(12):8885–8903. [DOI] [PubMed] [Google Scholar]
- 35. Bond DJ, Andreazza AC, Torres IJ, Honer WG, Lam RW, Yatham LN.. Association of total peripheral inflammation with lower frontal and temporal lobe volumes in early-stage bipolar disorder: a proof-of-concept study. J Affect Disord. 2022;319:229–234. [DOI] [PubMed] [Google Scholar]
- 36. Eleni P, Georgia P, Constantine P, et al. Functional brain imaging of speeded decision processing in Parkinson’s disease and comparison with Schizophrenia. Psychiatry Res Neuroimaging. 2021;314:111312. [DOI] [PubMed] [Google Scholar]
- 37. Roomruangwong C, Noto C, Kanchanatawan B, et al. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol. 2020;57(2):778–797. [DOI] [PubMed] [Google Scholar]
- 38. Barbe-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME.. The interplay between immunosenescence and age-related diseases. Semin Immunopathol. 2020;42(5):545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teissier T, Boulanger E, Cox LS.. Interconnections between inflammageing and immunosenescence during ageing. Cells. 2022;11(3):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamagata AS, Rizzo LB, Cerqueira RO, et al. Differential impact of obesity on CD69 expression in individuals with bipolar disorder and healthy controls. Mol Neuropsychiatry. 2018;3(4):192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shnayder NA, Khasanova AK, Strelnik AI, et al. Cytokine imbalance as a biomarker of treatment-resistant schizophrenia. Int J Mol Sci . 2022;23(19):11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Yin Y, Zhou Y, et al. The mediating effect of brain-derived neurotrophic factor levels on childhood trauma and psychiatric symptoms in patients with first-episode schizophrenia. Aust N Z J Psychiatry. 2022;56(7):828–835. [DOI] [PubMed] [Google Scholar]
- 43. Tian L, Hui CW, Bisht K, et al. Microglia under psychosocial stressors along the aging trajectory: consequences on neuronal circuits, behavior, and brain diseases. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:27–39. [DOI] [PubMed] [Google Scholar]
- 44. Kartalci S, Erbay LG, Zayman EP, et al. IL-4, TGF-β, NF-κB, and MPO levels patients with treatment resistant schizophrenia. Klinik Psikofarmakoloji Bulteni. 2016;26:S24. [Google Scholar]
- 45. Simsek S, Yildirim V, Cim A, Kaya S.. Serum IL-4 and IL-10 levels correlate with the symptoms of the drug-naive adolescents with first episode, early onset schizophrenia. J Child Adolesc Psychopharmacol. 2016;26(8):721–726. [DOI] [PubMed] [Google Scholar]
- 46. Santoro A, Bientinesi E, Monti D.. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. [DOI] [PubMed] [Google Scholar]
- 47. Sonar SA, Shaikh S, Joshi N, Atre AN, Lal G.. IFN-gamma promotes transendothelial migration of CD4(+) T cells across the blood-brain barrier. Immunol Cell Biol. 2017;95(9):843–853. [DOI] [PubMed] [Google Scholar]
- 48. Kunis G, Baruch K, Rosenzweig N, et al. IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain. 2013;136(Pt 11):3427–3440. [DOI] [PubMed] [Google Scholar]
- 49. Yeo IJ, Yun J, Son DJ, et al. Overexpression of transmembrane TNFalpha in brain endothelial cells induces schizophrenia-relevant behaviors. Mol Psychiatry. 2022;28:843–855. [DOI] [PubMed] [Google Scholar]
- 50. Duchatel RJ, Meehan CL, Harms LR, et al. Late gestation immune activation increases IBA1-positive immunoreactivity levels in the corpus callosum of adult rat offspring. Psychiatry Res. 2018;266:175–185. [DOI] [PubMed] [Google Scholar]
- 51. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273–1279. [DOI] [PubMed] [Google Scholar]
- 52. Zhang L, Xu S, Huang Q, Xu H.. N-acetylcysteine attenuates the cuprizone-induced behavioral changes and oligodendrocyte loss in male C57BL/7 mice via its anti-inflammation actions. J Neurosci Res. 2018;96(5):803–816. [DOI] [PubMed] [Google Scholar]
- 53. Sugimoto K, Kakeda S, Watanabe K, et al. Relationship between white matter integrity and serum inflammatory cytokine levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Transl Psychiatry. 2018;8(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Son S, Arai M, Miyata J, et al. Enhanced carbonyl stress and disrupted white matter integrity in schizophrenia. Schizophr Res. 2020;223:242–248. [DOI] [PubMed] [Google Scholar]
- 55. Mitelman SA, Buchsbaum MS, Christian BT, et al. Relationship between white matter glucose metabolism and fractional anisotropy in healthy and schizophrenia subjects. Psychiatry Res Neuroimaging. 2020;299:111060. [DOI] [PubMed] [Google Scholar]
- 56. Stauffer EM, Bethlehem RAI, Warrier V, et al. Grey and white matter microstructure is associated with polygenic risk for schizophrenia. Mol Psychiatry. 2021;26(12):7709–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zong X, Zhang Q, He C, et al. DNA methylation basis in the effect of white matter integrity deficits on cognitive impairments and psychopathological symptoms in drug-naive first-episode schizophrenia. Front Psychiatry. 2021;12:777407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lim J, Sohn H, Kwon MS, Kim B.. White matter alterations associated with pro-inflammatory cytokines in patients with major depressive disorder. Clin Psychopharmacol Neurosci. 2021;19(3):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang X, Guo Y, Jia L, et al. Altered levels of plasma inflammatory cytokines and white matter integrity in bipolar disorder patients with suicide attempts. Front Psychiatry. 2022;13:861881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shao Y, Peng H, Huang Q, Kong J, Xu H.. Quetiapine mitigates the neuroinflammation and oligodendrocyte loss in the brain of C57BL/6 mouse following cuprizone exposure for one week. Eur J Pharmacol. 2015;765:249–257. [DOI] [PubMed] [Google Scholar]
- 61. Mitelman SA, Torosjan Y, Newmark RE, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res. 2007;92(1-3):211–224. [DOI] [PubMed] [Google Scholar]
- 62. Chaves C, Marque CR, Maia-de-Oliveira JP, et al. Effects of minocycline add-on treatment on brain morphometry and cerebral perfusion in recent-onset schizophrenia. Schizophr Res. 2015;161(2-3):439–445. [DOI] [PubMed] [Google Scholar]
- 63. Ghanizadeh A, Dehbozorgi A, OmraniSigaroodi M, et al. Minocycline as add-on treatment decreases the negative symptoms of schizophrenia; a randomized placebo-controlled clinical trial. Recent Pat Inflamm Allergy Drug Discov. 2014;8(3):211–215. [DOI] [PubMed] [Google Scholar]
- 64. Velloso FJ, Wadhwa A, Kumari E, Carcea I, Gunal O, Levison SW.. Modestly increasing systemic interleukin-6 perinatally disturbs secondary germinal zone neurogenesis and gliogenesis and produces sociability deficits. Brain Behav Immun. 2022;101:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoang D, Xu Y, Lutz O, et al. Inflammatory subtypes in antipsychotic-naïve first-episode schizophrenia are associated with altered brain morphology and topological organization. Brain Behav Immun. 2022;100:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ellman LM, Deicken RF, Vinogradov S, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121(1-3):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Petrulli J, Kalish B, Nabulsi N, et al. Systemic inflammation enhances stimulant-induced striatal dopamine elevation. Transl Psychiatry. 2017;7(3):e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bettcher BM, Watson CL, Walsh CM, et al. Interleukin-6, age, and corpus callosum integrity. PLoS One. 2014;9(9):e106521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


