Fig. 3 |. Forward-looking treatment paradigms in advanced ALK+ lung cancer.
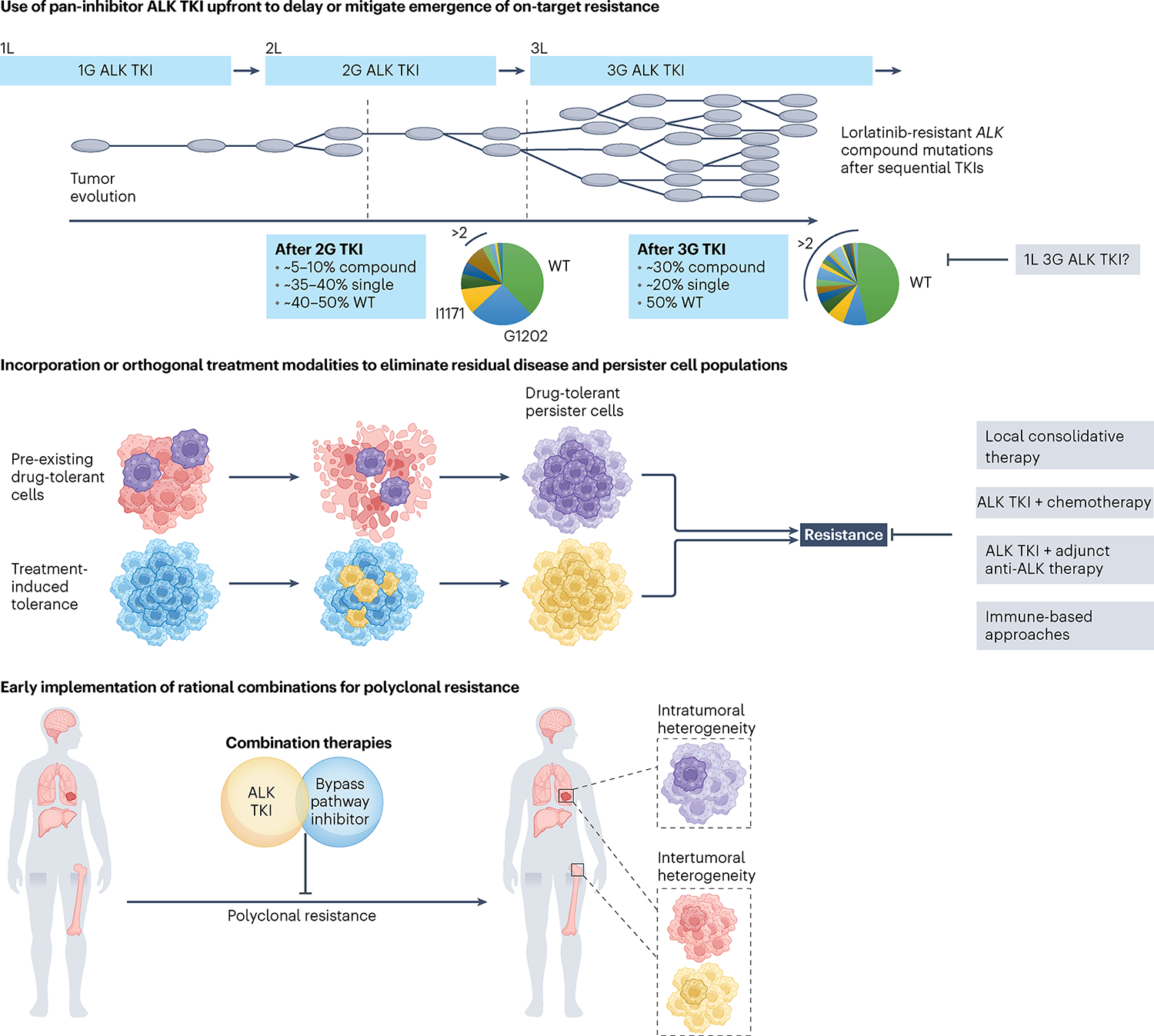
Top, sequential ALK TKI therapy culminating in lorlatinib induces compound ALK mutations, with ALKG1202R- or ALKI1171N-based compound mutations being the most common. The schematic depicts tumor clonal evolution with the multitude of single ALK mutations serving as a substrate for compound ALK mutations, highlighting the notion of stepwise accumulation of resistance mutations. Treatment with a highly potent pan-inhibitory third-generation ALK TKI in the first-line (1L) setting may allow for maximal cytoreduction and depth of response, limiting tumor heterogeneity that can emerge with less potent ALK TKIs. Middle, drug-tolerant cells that are present at the time of treatment may undergo expansion under therapeutic selective pressure, leading to treatment failure and clinical relapse. In parallel, persister cells that survive initial treatment may acquire de novo resistance alterations, serving as a nidus for the development of polyclonal resistance. Depicted in gray are potential adjunctive therapeutic strategies aimed at eliminating persister cells. Bottom, intratumoral heterogeneity can occur across different regions of the primary tumor and/or metastatic sites, with spatial heterogeneity represented by the presence of subclones with different genetic features. Intertumoral heterogeneity can also occur across different metastatic sites, which can be missed using single-site tissue biopsies. Studies are underway to evaluate the utility of early rational combinations and to stem polyclonal resistance. In parallel, efforts are ongoing to develop ultrasensitive diagnostic tools to track tumor response and detect microscopic disease. 2L, second line; 3L, third line.
