Abstract
Introduction
We investigated the safety, efficacy, functional, and clinical outcomes of intra-osseous implantation of mechanically isolated, autologous stromal vascular fraction (SVF), an Australian patented direct ultrasonication technology (Sahaj Therapy®) in osteonecrosis of the femoral head (ONFH).
Materials and Methods
A total of 32 cases of ONFH were enrolled in the study after confirming with an MRI of the affected hip. All cases were treated with an intra-osseous autologous SVF implantation [4–5 cc with the cellular dosage of 8.0 × 107 cells with a viability of > 85% SVF cells] on the same surgical sitting. All the cases were followed up clinically, functionally, and radiologically at regular intervals. A comparison of mean HOOS scores at different follow-ups was done using Paired ‘t’-test. A P value of < 0.05 was considered significant.
Results
In our study, male preponderance was seen (53.1%). According to the modified Ficat and Arlet classification, the most common grade of ONFH was grade 2 [right: 25 hips and left: 25 hips]. There was a statistically significant improvement in the mean HOOS score of the right hip (n = 10) and left hip (n = 9) from preoperative time till 72 months (P < 0.05). The follow-up MRI of the affected hips shows improved osteogenesis without any further worsening of the contour of the femoral head. No adverse effects were seen in any of the study participants.
Conclusion
For individuals with ONFH, treated with intra-osseous autologous SVF implantation in the same surgical procedure is an innovative and promising treatment modality. Even after 6 years of follow-up, the study participants with ONFH have shown good clinical and functional outcomes with autologous SVF.
Keywords: Adipose tissue, Stromal vascular fraction, Osteonecrosis, Femoral head
Introduction
Osteonecrosis of the femoral head (ONFH) is an advancing disease that is delineated by the spontaneous demise of the cellular element of the subchondral part of the bone as a result of loss of blood supply that causes osteoarthritis [1], the deficiency of blood supply can be because of various factors like trauma, genetic predilection, systemic abnormalities, metabolic components, drugs, and other local causes [2, 3]. The mechanism of development is not clear but the endpoint is reinstating the bone that is dead with thin fragile trabeculae that is susceptible to fracture and collapse with stress [1].
The pathogenesis behind ONFH remains ambiguous [4, 5], it is commonly perceived that the various causes predetermine the perilous blood supply to the femoral head, which in turn results in ischemia of the bone that triggers the demise of the cells of the bone and ultimately causes the collapse of the necrotic section of bone [6–8]. The sequel of the above-mentioned leads to the collapse of the femoral head and secondary arthritic changes of the affected hip [9, 10]. In a high number of patients, without early diagnosis and appropriate treatment, osteonecrosis progresses to collapse of the femoral head with the destruction of the hip joint and necessitates total hip arthroplasty (THA) to bring back effective function of the hip joint [11]. In the recent past, many young individuals are affected and THA at a young age will not increase the lifetime of the patient and so hip-conserving treatment options are mainly in need for these young individuals in the early stages of ONFH disease [12, 13].
Among the various available stem cells, the mesenchymal stromal cells (MSCs) are one of the most common subsets used, which is distributed in a vast range of tissues viz, peripheral circulation, adipose tissue, bone marrow, Wharton’s jelly, umbilical cord, amniotic fluid, dental pulp, hair follicle, dermal papillae, etc. [14–20]. MSCs can delineate into osteoblasts and endothelial cells to propagate the repair of bones and angiogenesis and also would crop growth factors to induce blood supply to the areas of necrosis by paracrine modes [21–24]. Stem cell therapy has been serving as one of the hip-conserving treatment choices for the past two decades since the first case report of stem cell treatment for ONFH [25]. In the past few years, there has been rapid progress in the field of tissue engineering and cell biotechnology, which has led to encouraging results in ONFH treatment.
Adipose tissue-derived mesenchymal stromal cells (AD-MSCs) are a good stem cell source for ONFH therapy and have many advantages such as easily attainable, greater productivity, and analogous potential for differentiation as bone marrow stem cells [26, 27]. The by-products of AD-MSCs are adipose tissue-derived stem cells (ADSC), microfat, nanofat, stromal vascular fraction, microvascular fragments, and exosomes [28]. The excessive expression of vascular endothelial growth factor (VEGF) could increase the osteogenesis capacity and neoangiogenesis by AD-MSCs [29]. In our study, we investigated the safety, efficacy, functional, and clinical outcomes of intra-osseous grafting of mechanically isolated, autologous stromal vascular fraction (SVF) Australian Patent technology (Sahaj Therapy®) in osteonecrosis of the femoral head (ONFH).
Materials and Methods
After obtaining the institutional ethical clearance [IMCHRC/IEC/2015/013 dated 02.05.2015], the present prospective observational study was conducted on 32 patients [from May 2015 to May 2021] suffering from osteonecrosis of the femoral head (ONFH) with modified Ficat and Arlet classification grades I to III. Voluntary written informed consent was obtained from the patient and/or his/her legally acceptable representative for participation in the study.
Inclusion Criteria
The patients with idiopathic ONFH of age between above 18 years, patients with modified Ficat and Arlet classification grades I to III ONFH, patients with self-reported difficulty in at least one of the following activities such as lifting and carrying groceries, walking 400 m, getting in and out of a chair, or going up or down the stairs, patients with an adequate renal (serum creatinine < 1.5 mg/dl), cardiovascular, and respiratory functions, patients with PT/INR < 1.5 and normal APTT value, and patients with an adequate immune system function and no known immunodeficiency were included in the study.
Exclusion Criteria
The patients with ages less than 18 years, patients with modified Ficat and Arlet classification grades IV ONFH and secondary ONFH, patients with an active neoplastic disease diagnosed in the last 3 years, patients with a BMI more than 35, patients with a history of any surgery including arthroscopy or major trauma to the affected hip joint in the last 12 months, patients with the signs of active infection or inflammation over the joint, patients with inflammatory joint diseases or laxity in joint, patients who are positive HIV, HbsAg, HCV or VDRL were excluded from the study.
Surgical Procedure
After obtaining consent for participation and other consents relevant to the treatment, the isolation and implantation of autologous SVF into hip joints were performed. The whole procedure from harvesting the autologous adipose tissue to the isolation and implantation of autologous SVF into the hip was done in the same surgical sitting which took around 90 min. The following steps were performed as depicted in Fig. 1.
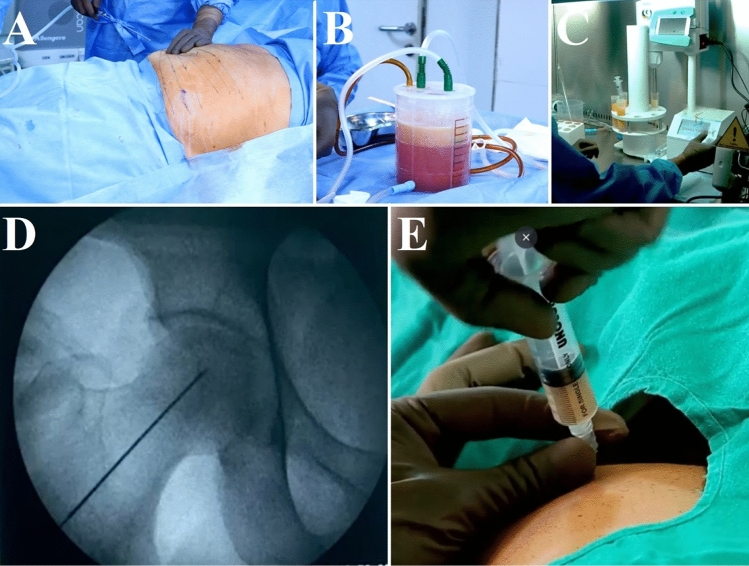
Fig. 1.
Preparation of stromal vascular fraction (SVF). A Marking of the abdomen for lipoaspiration, B lipoaspiration in the canister, C adipose tissue disruption by direct ultrasonic cavitation process, and D, E intra-osseous SVF grafting in same surgical sitting in the right femoral head
Lipoaspiration
The patient was prepared for the procedure with all aseptic precautions. Pre-procedural antibiotics, anxiolytics, and/or opioid-based pain medications were administered if needed. Under sedation/short general anesthesia, a stab incision on the abdomen using a #11 blade was made for cannula entry after local infiltration with 1% lidocaine with epinephrine 1:100,000. 40 ml of 2% lidocaine plus 1 ml of 1% epinephrine was added to a 1000 ml bag of 0.9% normal saline and was infiltrated with the tumescent anesthesia on the infraumbilical area of the abdomen. Approximately, 200–250 cc of adipose tissue was aspirated into the patented sterile Lipoaspiration Jar (Design No. 316580–001) containing 0.9% sterile normal saline and sodium bicarbonate.
Recovery of the Autologous Adipose Tissue (ACRU Unit)
Approximately 300–450 cc of autologous adipose tissue was harvested. In Class II Bio-Hood, the sample was divided into 50 ml tubes and tissue fragmentation was done using direct ultrasonic cavitation (Australian Patented Technology) which was used to separate the SVF or cellular fraction from fat. These 50 ml tubes were centrifuged with a pellet being formed at the bottom of the tube. The tube was turned upside down after screwing a tube filter to the 50 ml tube. The pellet separated was the SVF or cellular fraction, which is the heterogeneous population of cells. The cell count and viability were checked using the Muse Cell Flow cytometer.
Intra-osseous Autologous SVF Grafting
Under local anesthesia and mild sedation, chlorhexidine was used for hip preparation. Under c-arm guidance, a 18-gauge bone marrow needle was inserted into the necrotic area of the femoral and the prepared autologous SVF of 4–5 cc with the cellular dosage of 8.0 × 107 cells with a viability of > 85% SVF cells were implanted intraosseously into the affected femoral head under C-arm guidance.
Follow-up
Patients with autologous SVF implantation into the affected hips were instructed to contact the surgeon in case of fever, pain, and any other adverse events. Patients were followed up regularly functionally by Hip Disability and Osteoarthritis Outcome Score (HOOS) at the end of 3, 6, 12, 24, 36, 48, 60, and 72 months after the procedure by telephone, email, or in person for radiological documentation as depicted in Figs. 2, 3, 4 and 5. The patients were allowed to walk with support for first 3 weeks immediately after the surgical procedure and complete weight bearing was allowed after 3rd week in the post-operative period.
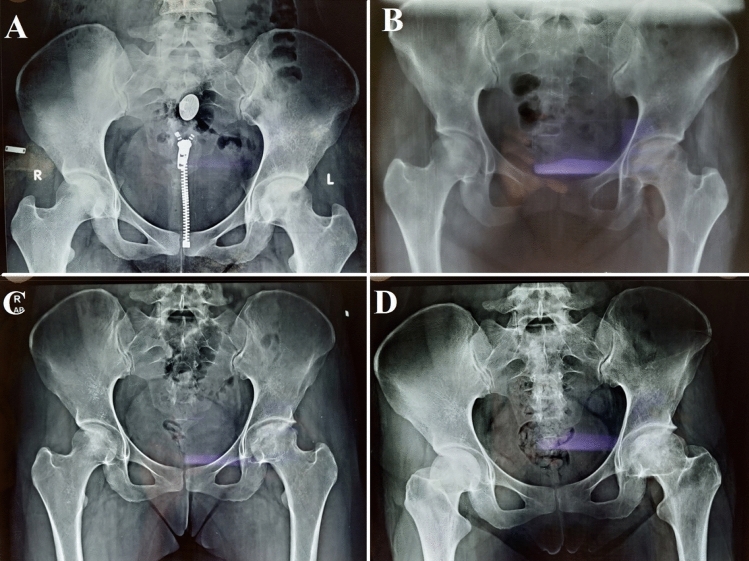
Fig. 2.
Plain radiograph of pelvis with bilateral hips (AP) showing A stage 2b left ONFH, B immediate follow-up x-ray of SVF implantation over the left femoral head, C 1-year follow-up x-ray with osteogenic activity over left femoral head, and D 4-year follow-up x-ray with maintained femoral head contour
Fig. 3.
A MRI of bilateral hips showing stage 2b left ONFH and B 4 years follow-up MRI of bilateral hips showing maintenance of left femoral head contour with improved osteogenesis
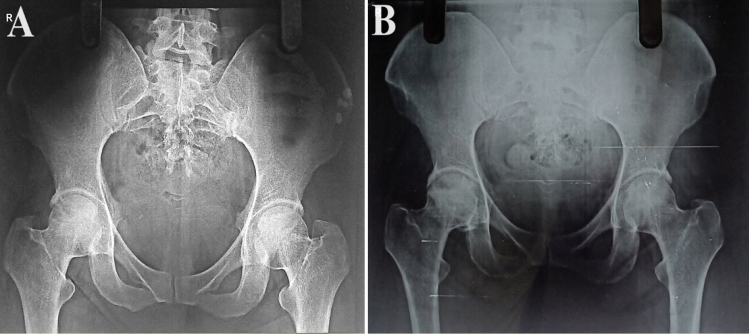
Fig. 4.
Plain radiograph of pelvis with bilateral hips (AP) showing A stage 2a ONFH of right hip and B 5-year follow-up x-ray with osteogenic activity over right femoral head with maintained femoral head contour
Fig. 5.
A–C MRI of bilateral hips showing stage 2a right ONFH and D–F 5 years follow-up MRI of bilateral hips showing maintenance of right femoral head contour with improved osteogenesis
Statistical Analysis
Statistical software, SPSS version 26.0, IBM Chicago, Illinois was used for statistical analysis. Descriptive statistics were presented as numbers and percentages. Paired ‘t’-test was applied to compare the preoperative HOOS score at various follow-ups. A P value of < 0.05 was taken as statistically significant.
Results
A total of 32 patients with ONFH treated with intra-osseous SVF implantation were analyzed and followed up at regular intervals. The demographic characteristics were tabulated in Table 1. The majority of the patients were in the age group 21–40 years (53.1%) and the least was more than 60 years age group (9.4%). The mean age of the patients was 41.97 ± 12.53 years (range: 21 to 62 years). Males were slightly more than the females in our series (53.1% vs. 46.9%). 43.8% of patients were in the normal weight group, 40.6% of patients were overweight and 12.5% of patients were obese. Patients with ONFH of 31 right hips (modified Ficat and Arlet classification: grade 1—4 hips; grade 2a—12 hips; grade 2b—13 hips, and grade 3—4 hips) and 28 left hips (modified Ficat and Arlet classification: grade 1—2 hips; grade 2a—15 hips; grade 2b—10 hips, and grade 3—1 hip) underwent autologous SVF implantation. The distribution of patients available at each follow-up is depicted in Table 2.
Table 1.
Demographic characteristics of study participants (N = 32)
| Demographic characteristics | Frequency | Percentage |
|---|---|---|
| Age | ||
| 21–40 years | 17 | 53.1 |
| 41–60 years | 12 | 37.5 |
| > 60 years | 3 | 9.4 |
| Gender | ||
| Female | 15 | 46.9 |
| Male | 17 | 53.1 |
| BMI category | ||
| Underweight | 1 | 3.1 |
| Normal weight | 14 | 43.8 |
| Overweight | 13 | 40.6 |
| Obese | 4 | 12.5 |
| Total | 32 | 100.0 |
Table 2.
Number of patients available at each follow-up (N = 32)
| Follow-up | Right hip | Left hip |
|---|---|---|
| 3 months | 31 | 28 |
| 6 months | 31 | 28 |
| 12 months | 27 | 24 |
| 24 months | 22 | 20 |
| 36 months | 20 | 18 |
| 48 months | 18 | 16 |
| 60 months | 15 | 14 |
| 72 months | 10 | 9 |
Preoperatively, the mean HOOS score of the right hip was 44.07 ± 10.39, at 3 months it was 55.82 ± 10.61, at 6 months it was 59.26 ± 9.75, at 12 months it was 65.06 ± 9.09, at 24 months it was 70.09 ± 9.42, at 36 months it was 73.71 ± 9.73, at 48 months it was 75.64 ± 9.51, at 60 months it was 77.27 ± 9.47 and at 72 months it was 79.65 ± 10.16 as mentioned in Fig. 6. There was a statistically significant improvement in the mean HOOS score of the right hip from preoperative time till 72 months (P < 0.05) as shown in Table 3.
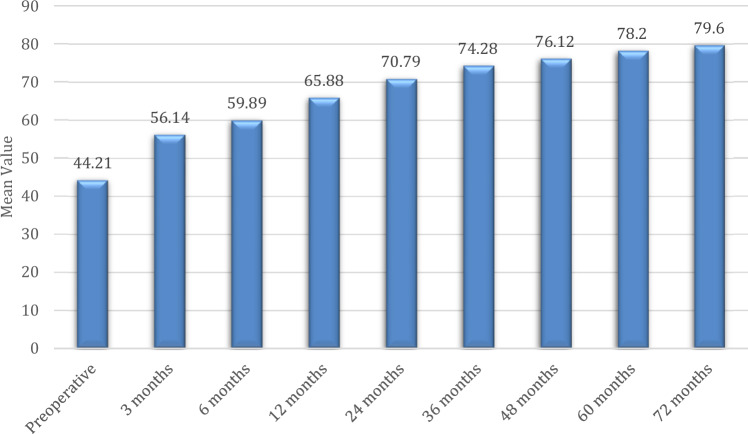
Fig. 6.
Comparison of mean HOOS score of the right hip at different time intervals
Table 3.
Comparison of mean HOOS score of the right hip at different time intervals (N = 31)
| Pair | Time interval | No | Mean ± SD | ‘t’ value, df | P value |
|---|---|---|---|---|---|
| Pair 1 | Preoperative | 31 | 44.07 ± 10.39 | − 6.542, df = 30 | 0.001* |
| 3 months | 31 | 55.82 ± 10.61 | |||
| Pair 2 | 3 months | 31 | 55.82 ± 10.61 | − 2.656, df = 30 | 0.013* |
| 6 months | 31 | 59.26 ± 9.75 | |||
| Pair 3 | 6 months | 27 | 60.68 ± 9.42 | − 6.884, df = 26 | 0.001* |
| 12 months | 27 | 65.06 ± 9.09 | |||
| Pair 4 | 12 months | 22 | 65.94 ± 9.51 | − 6.375, df = 21 | 0.001* |
| 24 months | 22 | 70.09 ± 9.42 | |||
| Pair 5 | 24 months | 20 | 70.29 ± 9.74 | − 7.880, df = 21 | 0.001* |
| 36 months | 20 | 73.71 ± 9.73 | |||
| Pair 6 | 36 months | 18 | 73.28 ± 10.16 | − 5.690, df = 17 | 0.001* |
| 48 months | 18 | 75.64 ± 9.51 | |||
| Pair 7 | 48 months | 15 | 75.67 ± 10.29 | − 2.762, df = 14 | 0.015* |
| 60 months | 15 | 77.27 ± 9.47 | |||
| Pair 8 | 60 months | 10 | 78.30 ± 10.82 | − 2.680, df = 9 | 0.025* |
| 72 months | 10 | 79.65 ± 10.16 |
Paired ‘t’ test was applied. P value < 0.05 was taken as statistically significant
Preoperatively, the mean HOOS score of the left hip was 44.21 ± 9.55, at 3 months it was 56.14 ± 8.40, at 6 months it was 59.89 ± 9.52, at 12 months it was 65.88 ± 8.95, at 24 months it was 70.79 ± 9.24, at 36 months it was 74.28 ± 9.51, at 48 months it was 76.12 ± 9.98, at 60 months it was 78.20 ± 8.28 and at 72 months it was 79.60 ± 8.16 as mentioned in Fig. 7. There was a statistically significant improvement in the mean HOOS score of the left hip from preoperative time till 72 months (P < 0.05) as shown in Table 4. None of the patients were deteriorated which have not necessitated the need for total hip replacement.
Fig. 7.
Comparison of mean HOOS score of the left hip at different time intervals
Table 4.
Comparison of mean HOOS score of the left hip at different time intervals (N = 28)
| Pair | Time interval | No | Mean ± SD | ‘t’ value, df | P value |
|---|---|---|---|---|---|
| Pair 1 | Preoperative | 28 | 44.21 ± 9.55 | − 10.353, df = 27 | 0.001* |
| 3 months | 28 | 56.14 ± 8.40 | |||
| Pair 2 | 3 months | 28 | 56.14 ± 8.40 | − 4.383, df = 27 | 0.001* |
| 6 months | 28 | 59.89 ± 9.52 | |||
| Pair 3 | 6 months | 24 | 61.41 ± 8.99 | − 7.527, df = 23 | 0.001* |
| 12 months | 24 | 65.88 ± 8.95 | |||
| Pair 4 | 12 months | 20 | 66.94 ± 9.16 | − 6.217, df = 19 | 0.001* |
| 24 months | 20 | 70.79 ± 9.24 | |||
| Pair 5 | 24 months | 18 | 71.36 ± 8.95 | − 6.296, df = 17 | 0.001* |
| 36 months | 18 | 74.28 ± 9.51 | |||
| Pair 6 | 36 months | 16 | 74.20 ± 9.77 | − 4.157, df = 15 | 0.001* |
| 48 months | 16 | 76.12 ± 9.98 | |||
| Pair 7 | 48 months | 14 | 76.07 ± 10.62 | − 2.305, df = 13 | 0.038* |
| 60 months | 14 | 78.20 ± 8.28 | |||
| Pair 8 | 60 months | 9 | 77.96 ± 9.49 | − 2.582, df = 8 | 0.033* |
| 72 months | 9 | 79.60 ± 8.16 |
Paired ‘t’ test was applied. P value < 0.05 was taken as statistically significant
Discussion
In the recent past, adult stem cell-based therapy has provided assuring results in increasing the formation of collateral vasculature in ischemic conditions [29, 30]. For a prolonged duration, the bone marrow-derived mesenchymal stromal cells (BM-MSCs) have been abundantly utilized for therapeutic purposes, which require neovascularization. They are progenitor cells with the property of multipotency, which is the ability to diversify into vascular cells and perpetuate neovascularization [30–32].
Other sources of easily accessible adult autologous stem cells include adipose tissue in adults that possess a high quantity of endothelial progenitor cells and MSCs with the property of multipotency [33]. Adipose tissue in the body has abundant vascularity and there is a major role in the remodeling process of the persistent vessels in the physiological behavior of the adipose tissue [34]. Zuk et al., for the first time in history, defined a group of look-alike cells that resembles the fibroblast cells in adipose tissue-derived SVF, which could disseminate in vitro into myogenic, osteogenic, chondrogenic, and adipogenic cells [35].
The contents of SVF include endothelial cells (ECs), smooth muscle cells, fibroblasts, mural cells, macrophages, smooth muscle cells, and MSCs/phenotypes of other progenitor cells [28, 36]. Of these, the focus has been kept on the characteristic features and functional capability of AD-MSCs in the mechanism of neovascularization. Though ADSCs have competency in the field of regenerative and reconstructive medicine, SVF has proven to have exceptional results in comparison to only AD-MSC therapy [37]. The unique feature of SVF cells is their capability to gather into a hierarchical, divided, infused vasculature in vivo [36].
The ECs and their precursors have CD31 as their prototype surface marker and CD34 is manifested by the duo-the ECs and hematopoietic stem/precursor cells [38]. Klar et al.[39] separated the SVF cellular components into CD34+/CD31+ cells and CD34+/CD31− cells by the technique of flow cytometry. Their observation lead to the findings viz CD31+ cells comprised of only 25% of the total cell content in SVF and they express a particular endothelial phenotype that could assert CD31 and vascular endothelial growth factor receptor 2 (VEGFR2) and can assimilate fluorescent labeled acetylated low-density lipoprotein. On the other hand, the CD31- cells separated from the human adipose tissue displayed properties of stromal stem cells, exhibited surface markers specific to MSCs, and showed positivity for CD90 and vimentin [39]. Morris et al. have found that myeloid cells of mice comprise 22% of SVF cells [40].
In the initiation of osteogenic differentiation, transplantation of autologous AD-MSCs can increase osteogenesis and elevate bone density and bone mass in ONFH [41]. The excessive expression of VEGF can propagate osteogenesis and angiogenesis by AD-MSCs [29]. Abudusaimi et al. stated that core decompression along with transplantation of AD-MSCs enhanced the osteogenic potential of the necrotic patches by enhancing the osteocalcin expression of ONFH [42]. Pak et al. injected autologous adipose SVF into the hip by percutaneous means and observed that there was an improvement in the symptoms & there was a promotion of bone regeneration in the osteonecrotic areas [43].
It is easy to obtain adipose tissue-derived MSCs and have some similar biological features and properties to BM-MSCs, also enormous amounts of MSCs can be obtained without any necessity for ex-vivo culture [44]. There are various comments that the ischemic surrounding reduces the osteoprogenitor cell count in the healthy part of the head of the femur which in turn provides the process of necrosis an upper hand and leads to the spread of the lesion because of the inability to repair the disease progress [43]. Lee et al. elaborated on the potentiality for the repair of the human AD-MSCs, which when implanted into mice by activating an excessive amount of osteoblasts and osteoclasts activeness in the bone, and inferred that AD-MSCs would be a helpful tool in the treatment of osteoporosis and repair of bones [45]. Jeyaraman et al. concluded that a combination of core decompression with BM-MSCs implantation provides better osteogenesis at the necrosis of the femoral head than core decompression alone [21].
From recent studies, it has been proven that the potential of differentiation and repair of the AD-MSCs are equivalent to the mandible-derived MSCs (MD-MSCs) with actual dominance in regards to culture, harvest, and interventional safety [46]. Researchers are working on the details regarding the optimization of the treatment modalities in bone lesions and defects. Mihaila et al. conducted a study that demonstrates the fact that by acquiring the needful amount of subpopulation of adipose tissue of humans, we will be able to procure the relevant type of cells needed for the manufacturing of vascularized bone tissue-engineered scaffolds, whereas Behr et al. delineated the fact that invitro employment of particular growth factors may improve the osteogenic potential of AD-MSCs [47].
Core decompression, often with augmentation, stands as a widely accepted treatment for early-stage ONFH. The overall success rate is 65% and varies widely with the success rate showing significant difference on the outcomes of different stages [48]. Core decompression predominantly aims to relieve bone pressure. In contrast, our approach capitalizes on the regenerative potential harbored within adipose-derived stem cells from the stromal vascular fraction. These cells exhibit promising attributes in fostering tissue rejuvenation, dampening inflammation, and facilitating angiogenesis. Despite its relative minimally invasive nature, core decompression involves drilling and can entail a protracted recovery span (typically around 6 weeks before full weight-bearing is advised). Conversely, the SVF technique's less invasive procedure involves adipose tissue extraction, subsequent SVF processing, and targeted injection into the afflicted region, yielding briefer recuperation periods. Notably, our technique even permits immediate weight-bearing. This study underscores the distinct edge of the SVF approach. Choosing between the two methodologies pivots on factors like the patient's status, inclinations, and disease stage. Enhanced comprehension of the comparative efficacy in tackling ONFH necessitates additional research and comprehensive clinical trials.
In our study, there was a statistically significant improvement in the mean HOOS score of the right hip (n = 10) and left hip (n = 9) from preoperative time till 72 months (P < 0.05). The follow-up MRI of the affected hips shows improved osteogenesis without any further worsening of the contour of the femoral head. No adverse effects were seen in any of the study participants. The limitations of the study are the small sample size of study participants with a shorter duration of follow-up, only 20% of the study participants were followed-up for 6 years, and no comparison group to compare the effectiveness of autologous SVF in ONFH. We recommend a large blinded controlled trial to validate the results of our study.
Further research is essential to comprehensively unravel the precise mechanisms of SVF's action, as these potential actions hold promise in contributing to the alleviation of ONFH. The potential mechanisms of action could include:
The ability of MSCs to differentiate within SVF into various cell types, including bone-forming cells (osteoblasts), aiding in the formation of new bone tissue and repairing the damaged bone,
Beyond their direct differentiation potential, the MSCs within SVF secrete various bioactive molecules through paracrine signaling. These molecules encompass growth factors, cytokines, and extracellular vesicles, which can significantly impact local cells, promoting their survival, proliferation, and regeneration, and
Inflammation is often intertwined with ONFH, exacerbating tissue damage. SVF also contains anti-inflammatory cytokines that can modulate the immune response and mitigate inflammation in the affected region. This reduction in inflammation can foster an environment supportive of tissue recovery.
Conclusions
In our study, significant improvements in HOOS scores and enhanced osteogenesis, evident in postoperative MRI, underscore the positive impact of SVF treatment on ONFH over a 6-year follow-up period. The absence of adverse effects among participants is encouraging. Nevertheless, the study's limitations, such as a small sample size and short follow-up duration for some participants, call for a larger, controlled trial to validate our findings and better compare the effectiveness of autologous SVF therapy in ONFH. This research direction holds the potential for SVF to become one tool in orthopedics approach to treatment of OFNH.
Author Contributions
Conceptualization—VT, RS; Data procurement—MBP, AKS; Data analysis—RS; Manuscript writing—MJ, AN; Manuscript revision—MJ, AN; and Project administration—VT, RS. All authors agreed to publish the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
IEC approval
IMCHRC/IEC/2015/013 dated 02.05.2015.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lespasio MJ, Sodhi N, Mont MA. Osteonecrosis of the hip: A primer. The Permanente Journal. 2019;23:18–100. doi: 10.7812/TPP/18-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moghamis I, Alhammoud AA, Kokash O, Alhaneedi GA. The outcome of hyperbaric oxygen therapy versus core decompression in the non-traumatic avascular necrosis of the femoral head: Retrospective cohort study. Annals of Medicine and Surgery. 2021;62:450–454. doi: 10.1016/j.amsu.2021.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CC, Greenspan A, Gershwin ME. Osteonecrosis: Current perspectives on pathogenesis and treatment. Seminars in Arthritis and Rheumatism. 1993;23(1):47–69. doi: 10.1016/s0049-0172(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 4.Marker DR, Seyler TM, Ulrich SD, Srivastava S, Mont MA. Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clinical Orthopaedics and Related Research. 2008;466(5):1093–1103. doi: 10.1007/s11999-008-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones KB, Seshadri T, Krantz R, Keating A, Ferguson PC. Cell-based therapies for osteonecrosis of the femoral head. Biology of Blood and Marrow Transplantation. 2008;14(10):1081–1087. doi: 10.1016/j.bbmt.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Cardozo JB, Andrade DMS, Santiago MB. The use of bisphosphonate in the treatment of avascular necrosis: A systematic review. Clinical Rheumatology. 2008;27(6):685–688. doi: 10.1007/s10067-008-0861-9. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, et al. Osteonecrosis of the hip: Management in the 21st century. Instructional Course Lectures. 2003;52:337–355. [PubMed] [Google Scholar]
- 8.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. The Journal of Bone and Joint Surgery. American Volume. 1995;77(3):459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: Ten years later. The Journal of Bone and Joint Surgery. American Volume. 2006;88(5):1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 10.Fan M, Peng J, Qin L, Lu S. Experimental animal models of osteonecrosis. Rheumatology International. 2011;31(8):983–994. doi: 10.1007/s00296-011-1819-9. [DOI] [PubMed] [Google Scholar]
- 11.Hernigou P, Poignard A, Nogier A, Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. The Journal of Bone and Joint Surgery. American Volume. 2004;86(12):2589–2593. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) The New England Journal of Medicine. 1992;326(22):1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 13.Jones JP. Coagulopathies and osteonecrosis. Acta Orthopaedica Belgica. 1999;65(Suppl 1):5–8. [PubMed] [Google Scholar]
- 14.Jeyaraman N, Prajwal GS, Jeyaraman M, Muthu S, Khanna M. Chondrogenic potential of dental-derived mesenchymal stromal cells. Osteology. 2021;1(3):149–174. doi: 10.3390/osteology1030016. [DOI] [Google Scholar]
- 15.Jeyaraman M, Muthu S, Ganie PA. Does the source of mesenchymal stem cell have an effect in the management of osteoarthritis of the knee? Meta-analysis of randomized controlled trials. Cartilage. 2021;13(1 Suppl):1532S–1547S. doi: 10.1177/1947603520951623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: A review. American Journal of Translational Research. 2021;13(2):448–461. [PMC free article] [PubMed] [Google Scholar]
- 17.Muthu S, Patil SC, Jeyaraman N, Jeyaraman M, Gangadaran P, Rajendran RL, et al. Comparative effectiveness of adipose-derived mesenchymal stromal cells in the management of knee osteoarthritis: A meta-analysis. World Journal of Orthopedics. 2023;14(1):23–41. doi: 10.5312/wjo.v14.i1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthu S, Jeyaraman M, Jain R, Gulati A, Jeyaraman N, Prajwal GS, et al. Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees—A panoramic review. Stem Cell Investigation. 2021;8:13. doi: 10.21037/sci-2020-055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeyaraman M, Verma T, Jeyaraman N, Patro BP, Nallakumarasamy A, Khanna M. Is mandible derived mesenchymal stromal cells superior in proliferation and regeneration to long bone-derived mesenchymal stromal cells? World Journal of Methodology. 2023;13(2):10–17. doi: 10.5662/wjm.v13.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyaraman M, Muthu S, Gangadaran P, Ranjan R, Jeyaraman N, Prajwal GS, et al. Osteogenic and chondrogenic potential of periosteum-derived mesenchymal stromal cells: Do they hold the key to the future? Pharmaceuticals. 2021;14(11):1133. doi: 10.3390/ph14111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeyaraman M, Muthu S, Jain R, Khanna M. Autologous bone marrow derived mesenchymal stem cell therapy for osteonecrosis of femoral head: A systematic overview of overlapping meta-analyses. Journal of Clinical Orthopaedics and Trauma. 2021;13:134–142. doi: 10.1016/j.jcot.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H-S, Huang G-T, Chiang H, Chiou L-L, Chen M-H, Hsieh C-H, et al. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells (Dayton, Ohio) 2003;21(2):190–199. doi: 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Li G, Liu M, Zhou T, Zhou H. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. Journal of Bioscience and Bioengineering. 2016;121(2):213–219. doi: 10.1016/j.jbiosc.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Haumer A, Bourgine PE, Occhetta P, Born G, Tasso R, Martin I. Delivery of cellular factors to regulate bone healing. Advanced Drug Delivery Reviews. 2018;129:285–294. doi: 10.1016/j.addr.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clinical Orthopaedics and Related Research. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Wyles CC, Houdek MT, Crespo-Diaz RJ, Norambuena GA, Stalboerger PG, Terzic A, et al. Adipose-derived mesenchymal stem cells are phenotypically superior for regeneration in the setting of osteonecrosis of the femoral head. Clinical Orthopaedics and Related Research. 2015;473(10):3080–3090. doi: 10.1007/s11999-015-4385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8(2):166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Muthu S, Jeyaraman M, Ranjan R, Jha SK. Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World Journal of Stem Cells. 2021;13(10):1360–1381. doi: 10.4252/wjsc.v13.i10.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H-J, Cai B, Zhao X-Y, Li S-Q, Feng W, Liu J-G, et al. Repairing diabetic rats with bone defect by VEGF165 gene modified adipose-derived stem cells. China Journal of Orthopaedics and Traumatology. 2017;30(6):545–551. doi: 10.3969/j.issn.1003-0034.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells (Dayton, Ohio) 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 31.Silva GV, Litovsky S, Assad JAR, Sousa ALS, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 32.Janeczek PK, Leferink A, Groen N, Fernandes H, Moroni L, van Blitterswijk C, et al. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE. 2012;7(10):e46842. doi: 10.1371/journal.pone.0046842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry. Part A: The Journal of the International Society for Analytical Cytology. 2010;77(1):22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y. Angiogenesis modulates adipogenesis and obesity. The Journal of Clinical Investigation. 2007;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 36.Ramakrishnan VM, Boyd NL. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Engineering. Part B, Reviews. 2018;24(4):289–299. doi: 10.1089/ten.teb.2017.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Research & Therapy. 2017;8(1):145. doi: 10.1186/s13287-017-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics (IFATS) and Science and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klar AS, Güven S, Zimoch J, Zapiórkowska NA, Biedermann T, Böttcher-Haberzeth S, et al. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatric Surgery International. 2016;32(1):17–27. doi: 10.1007/s00383-015-3808-7. [DOI] [PubMed] [Google Scholar]
- 40.Morris ME, Beare JE, Reed RM, Dale JR, LeBlanc AJ, Kaufman CL, et al. Systemically delivered adipose stromal vascular fraction cells disseminate to peripheral artery walls and reduce vasomotor tone through a CD11b+ cell-dependent mechanism. Stem Cells Translational Medicine. 2015;4(4):369–380. doi: 10.5966/sctm.2014-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aimaiti A, Saiwulaiti Y, Saiyiti M, Wang Y-H, Cui L, Yusufu A. Therapeutic effect of osteogenically induced adipose derived stem cells on vascular deprivation-induced osteonecrosis of the femoral head in rabbits. Chinese Journal of Traumatology. 2011;14(4):215–220. [PubMed] [Google Scholar]
- 42.Abudusaimi A, Aihemaitijiang Y, Wang Y-H, Cui L, Maimaitiming S, Abulikemu M. Adipose-derived stem cells enhance bone regeneration in vascular necrosis of the femoral head in the rabbit. The Journal of International Medical Research. 2011;39(5):1852–1860. doi: 10.1177/147323001103900528. [DOI] [PubMed] [Google Scholar]
- 43.Pak J. Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician. 2012;15(1):75–85. doi: 10.36076/ppj.2012/15/75. [DOI] [PubMed] [Google Scholar]
- 44.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells and Development. 2012;21(14):2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Kim H, Kim J-M, Kim J-R, Kim K-J, Kim Y-J, et al. Systemic transplantation of human adipose-derived stem cells stimulates bone repair by promoting osteoblast and osteoclast function. Journal of Cellular and Molecular Medicine. 2011;15(10):2082–2094. doi: 10.1111/j.1582-4934.2010.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schäffler A, Büchler C. Concise review: Adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells (Dayton, Ohio) 2007;25(4):818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 47.Behr B, Tang C, Germann G, Longaker MT, Quarto N. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells (Dayton, Ohio) 2011;29(2):286–296. doi: 10.1002/stem.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua K-C, Yang X-G, Feng J-T, Wang F, Yang L, Zhang H, et al. The efficacy and safety of core decompression for the treatment of femoral head necrosis: A systematic review and meta-analysis. Journal of Orthopaedic Surgery and Research. 2019;14(1):306. doi: 10.1186/s13018-019-1359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]