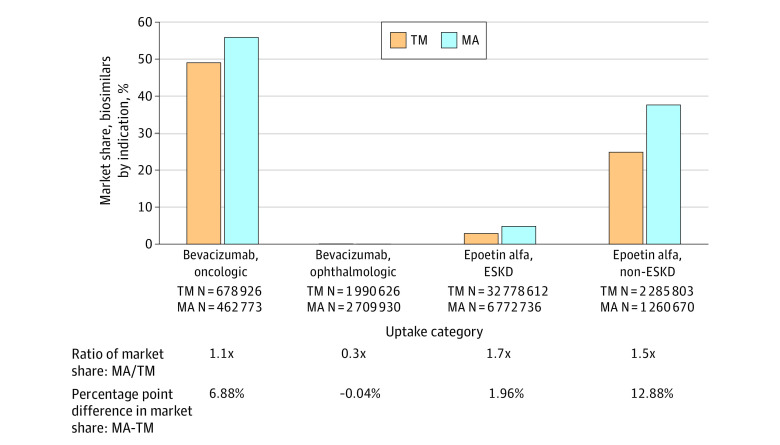
Figure 2. Cumulative Percentage Market Share of Bevacizumab and Epoetin Alfa Biosimilars Through 36 Months After Biosimilar Introduction, Stratified by Indication and Traditional Medicare (TM) vs Medicare Advantage (MA).
The percentage of the market share held by all aggregated biosimilar products out of all bevacizumab and epoetin alfa product usage through the first 36 months after biosimilar introduction, stratified by TM vs MA. Bevacizumab usage is stratified by ophthalmic and oncologic indications, using ophthalmic-related and oncologic-related keywords in the administrations’ corresponding primary diagnoses codes to categorize administrations (approximately 2% of total administrations did not have either type of keyword and were excluded). Epoetin alfa usage is stratified by end-stage kidney disease (ESKD) with dialysis vs non-ESKD, using administrations’ Healthcare Common Procedure Coding System code description to categorize administrations.