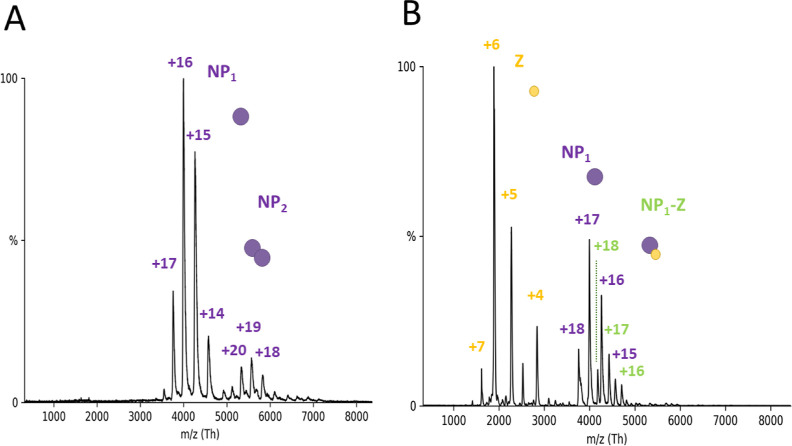
Figure 3.
NP-Z interaction is independent of the oligomerization state of the NP in nMS. (A) NP trimerization mutant R52A was measured with nMS in a 150 mM ammonium acetate solution at pH 7.5 and a concentration of 5.2 μM. Acceleration voltage in the collision cell was at 50 V for the depicted spectrum. R52A appears primarily in the monomeric state between 3500 and 4500 with charge states between +17 and +14 and a corresponding mass of 63.90 ± 0.08 kDa. A small fraction of dimeric NP is visible in the m/z range between 5000 and 6000 with the corresponding charge state of +20, +19, and +18. The most abundant species was normalized to 1. (B) R52A and Z were incubated in a 1:3 ratio and measured with the same instrument settings as described in A. Spectra show new peak series appearing for unbound Z protein in the m/z range between 1500 and 3000. New peaks also appear in the range between 4000 and 5000 m/z with +18, +17, and +16 charge states corresponding to a complexation of R52A and Z (75.5 ± 0.3 kDa).