Abstract
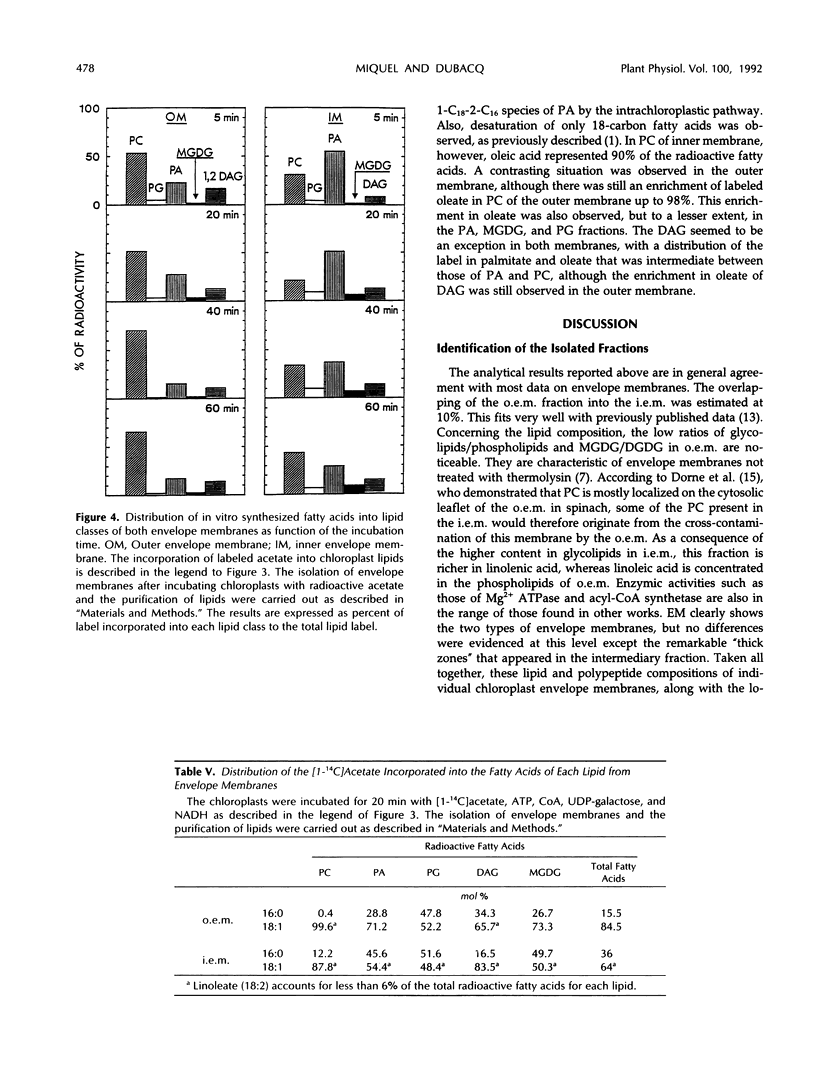
When incubated with [1-14C]acetate and cofactors (ATP, Coenzyme A, sn-glycerol-3-phosphate, UDPgalactose, and NADH), intact chloroplasts synthesized fatty acids that were subsequently incorporated into most of the lipid classes. To study lipid synthesis at the chloroplast envelope membrane level, 14C-labeled pea (Pisum sativum) chloroplasts were subfractionated using a single flotation gradient. The different envelope membrane fractions were characterized by their density, lipid and polypeptide composition, and the localization of enzymic activities (UDPgalactose-1,2 diacylglycerol galactosyltransferase, Mg2+-dependent ATPase). They were identified as very pure outer membranes (light fraction) and strongly enriched inner membranes (heavy fraction). A fraction of intermediate density, which probably contained double membranes, was also isolated. Labeled glycerolipids recovered in the inner envelope membrane were phosphatidic acid, phosphatidyl-glycerol, 1,2 diacylglycerol, and monogalactosyldiacylglycerol. Their 14C-fatty acid composition indicated that a biosynthetic pathway similar to the prokaryotic pathway present in cyanobacteria occurred in the inner membrane. In the outer membrane, phosphatidylcholine was the most labeled glycerolipid. Phosphatidic acid, phosphatidylglycerol, 1,2 diacylglycerol, and monogalactosyldiacylglycerol were also labeled. The 14C-fatty acid composition of these lipids showed a higher proportion of oleate than palmitate. This labeling, different from that of the inner membrane, could result either from transacylation activities or from a biosynthetic pathway not yet described in pea and occurring partly in the outer chloroplast envelope membrane. This metabolism would work on an oleate-rich pool of fatty acids, possibly due to the export of oleate from chloroplast toward the extrachloroplastic medium. The respective roles of each membrane for chloroplast lipid synthesis are emphasized.
Full text
PDF


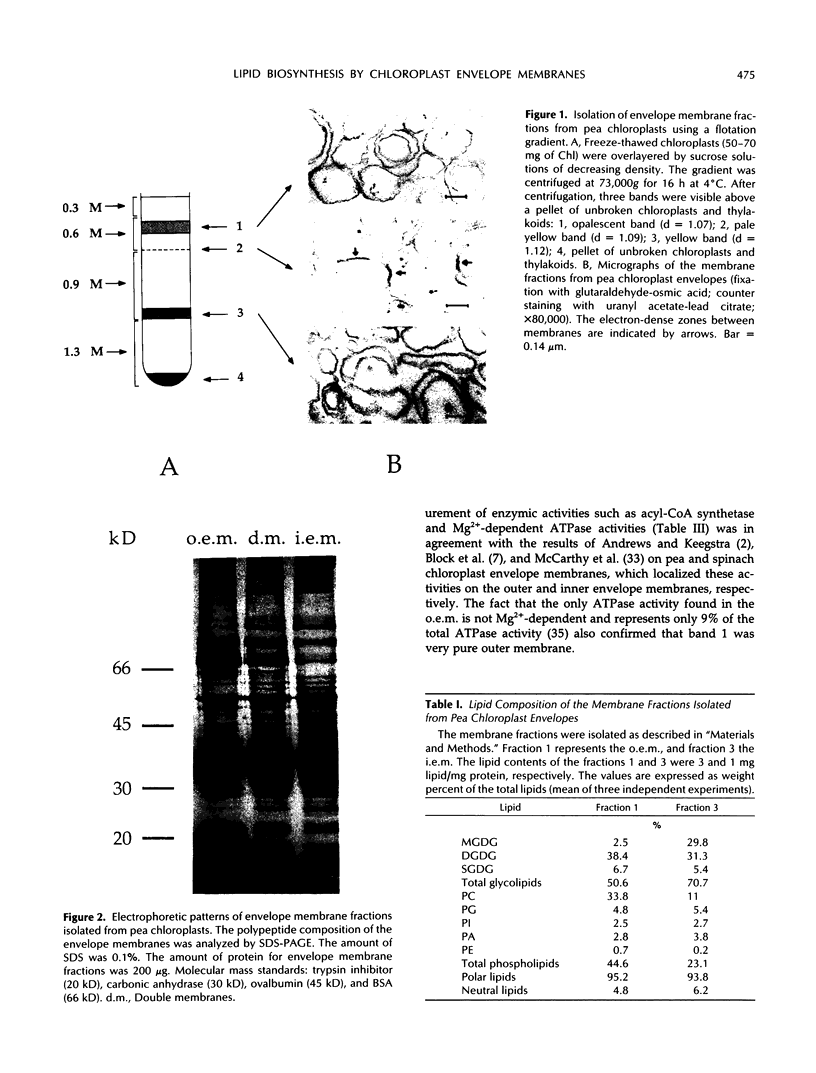
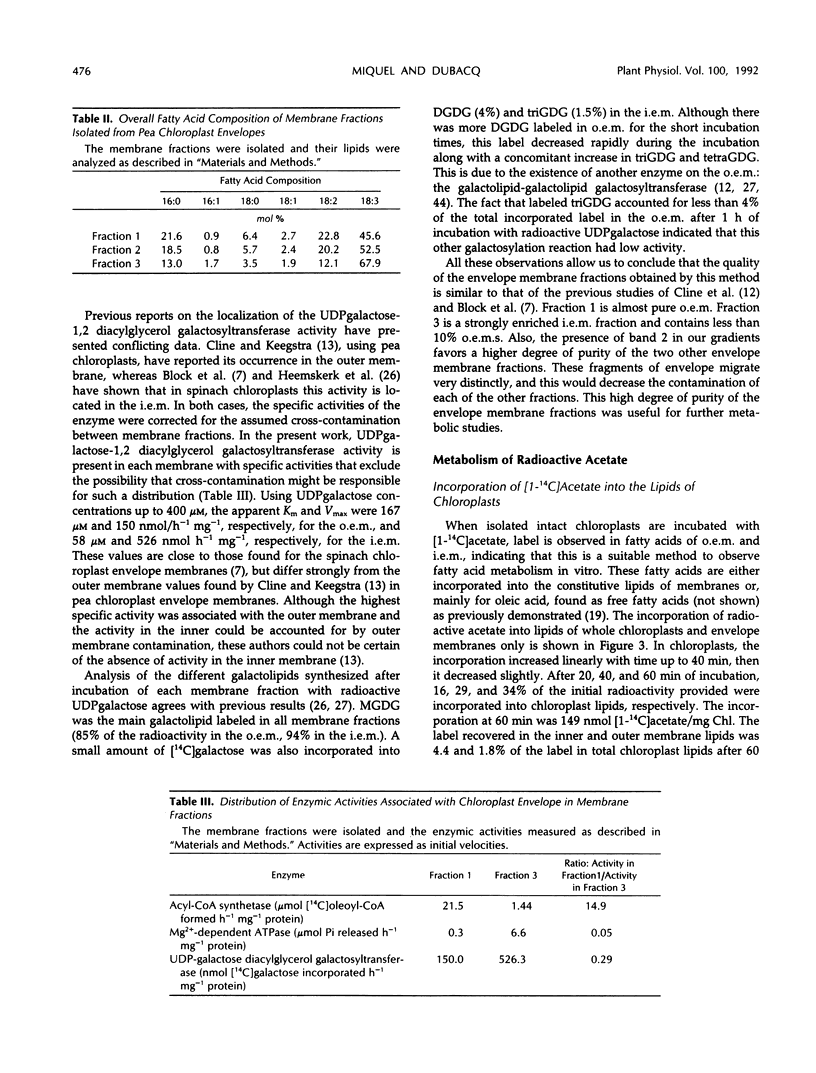



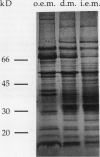
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J., Keegstra K. Acyl-CoA Synthetase Is Located in the Outer Membrane and Acyl-CoA Thioesterase in the Inner Membrane of Pea Chloroplast Envelopes. Plant Physiol. 1983 Jul;72(3):735–740. doi: 10.1104/pp.72.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J., Mudd J. B. Phosphatidylglycerol synthesis in pea chloroplasts: pathway and localization. Plant Physiol. 1985 Sep;79(1):259–265. doi: 10.1104/pp.79.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J., Ohlrogge J. B., Keegstra K. Final step of phosphatidic Acid synthesis in pea chloroplasts occurs in the inner envelope membrane. Plant Physiol. 1985 Jul;78(3):459–465. doi: 10.1104/pp.78.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem. 1983 Nov 10;258(21):13281–13286. [PubMed] [Google Scholar]
- Browse J., Warwick N., Somerville C. R., Slack C. R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the '16:3' plant Arabidopsis thaliana. Biochem J. 1986 Apr 1;235(1):25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Keegstra K. Galactosyltransferases involved in galactolipid biosynthesis are located in the outer membrane of pea chloroplast envelopes. Plant Physiol. 1983 Feb;71(2):366–372. doi: 10.1104/pp.71.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorne A. J., Joyard J., Block M. A., Douce R. Localization of phosphatidylcholine in outer envelope membrane of spinach chloroplasts. J Cell Biol. 1985 May;100(5):1690–1697. doi: 10.1083/jcb.100.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Joyard J. Biochemistry and function of the plastid envelope. Annu Rev Cell Biol. 1990;6:173–216. doi: 10.1146/annurev.cb.06.110190.001133. [DOI] [PubMed] [Google Scholar]
- Douce R. Site of biosynthesis of galactolipids in spinach chloroplasts. Science. 1974 Mar 1;183(4127):852–853. doi: 10.1126/science.183.4127.852. [DOI] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G., Browse J. Glycerolipid labelling kinetics in isolated intact chloroplasts. Biochem J. 1984 Dec 1;224(2):637–643. doi: 10.1042/bj2240637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G. Relationship between fatty-acyl composition of diacylgalactosylglycerol and turnover of chloroplast phosphatidate. Biochem J. 1983 Mar 15;210(3):949–952. doi: 10.1042/bj2100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra D. J., Ohlrogge J. B., Frentzen M. Activity of acyl carrier protein isoforms in reactions of plant Fatty Acid metabolism. Plant Physiol. 1986 Oct;82(2):448–453. doi: 10.1104/pp.82.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R., Siebertz H. P., Heinz E. Distribution of radioactive lipids between envelopes and thylakoids from chloroplasts labelled in vivo. Eur J Biochem. 1980;108(1):171–176. doi: 10.1111/j.1432-1033.1980.tb04709.x. [DOI] [PubMed] [Google Scholar]
- Joyard J., Stumpf P. K. Synthesis of Long-Chain Acyl-CoA in Chloroplast Envelope Membranes. Plant Physiol. 1981 Feb;67(2):250–256. doi: 10.1104/pp.67.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCarty D. R., Keegstra K., Selman B. R. Characterization and localization of the ATPase associated with pea chloroplast envelope membranes. Plant Physiol. 1984 Nov;76(3):584–588. doi: 10.1104/pp.76.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Shine W. E., Stumpf P. K. Fat metabolism in higher plants. Characterization of plant acyl-ACP and acyl-CoA hydrolases. Arch Biochem Biophys. 1978 Aug;189(2):382–391. doi: 10.1016/0003-9861(78)90225-4. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Long-chain acyl-coenzyme A synthetase activity of spinach chloroplasts is concentrated in the envelope. Biochem J. 1977 Feb 15;162(2):457–459. doi: 10.1042/bj1620457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H., Heinz E. Desaturation of oleoyl groups in envelope membranes from spinach chloroplasts. Proc Natl Acad Sci U S A. 1990 Dec 1;87(23):9477–9480. doi: 10.1073/pnas.87.23.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Besouw A., Wintermans J. F. Galactolipid formation in chloroplast envelopes. I. Evidence for two mechanisms in galactosylation. Biochim Biophys Acta. 1978 Apr 28;529(1):44–53. doi: 10.1016/0005-2760(78)90102-9. [DOI] [PubMed] [Google Scholar]