Abstract
Cardiovascular disease, predominantly acute coronary syndrome (ACS), is the leading cause of death for both men and women. For decades, this has been a global healthcare challenge. The main reason for thrombus formation in the coronary arteries is platelet accumulation as part of an inflammatory reaction. The efforts to combat this process of platelet aggregation have led researchers to discover antiplatelet drugs, which have been a keystone in treating cardiovascular diseases related to arterial thrombus formation. Antiplatelet drugs inhibit various platelet responses and help mitigate atherothrombosis, thereby playing a major role in both primary and secondary prevention of ACS.
This study employs a scoping review approach to recapitulate the data in the existing literature regarding the efficacy and safety of antiplatelet therapy in patients with ACS. By searching a total of 14,882 journals that were published between 2013 and July 26, 2023, 10 papers were selected for in-depth analysis. We conducted this literature search by using PubMed and Google Scholar databases and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the corresponding PRISMA Extension for Scoping Reviews in performing this review.
The review findings revealed that the current approach of using antiplatelet agents in ACS is safe and efficient, provided that bleeding risk assessment is conducted and any prior contraindications are recognized before administering the drugs.
Ethical approval was not required for this review as it involved secondary data collection from published journals. The findings of this scoping review will be published in peer-reviewed journals and presented at conferences.
Keywords: efficacy and safety, heart attack, acs ( acute coronary syndrome ), dual-antiplatelet therapy (dapt), antiplatelet drugs, antiplatelet therapy
Introduction and background
Ischemic heart disease (IHD) and acute coronary syndrome (ACS) are among the primary causes of death at a younger and economically productive age in low- and middle-income countries (LMICs) than in high-income nations [1]. ACS is the primary cause of death in the Asia-Pacific region, accounting for more than 50% of the global burden [2]. The root cause of coronary artery disease (CAD) has been proven to be atherosclerosis, a chronic inflammatory process [3]. Arterial thrombosis following atherosclerotic plaque rupture or erosion is the primary trigger of ACS [4,5].
ACS occurs when blood flow to the heart muscle gets interrupted. ACS can occur if a blockage forms in the coronary artery, either partially or fully. Based on electrocardiogram (ECG) results, acute myocardial infarction (AMI) is classified into two types: ST-segment elevated myocardial infarction (STEMI) and non-ST-segment elevated myocardial infarction (NSTEMI). A complete coronary artery occlusion causes STEMI, whereas NSTEMI patients have a partial blockage and no apparent ST-elevation on their ECG. STEMI patients are at a higher risk of death than NSTEMI patients [6]. Platelet adhesion, activation, and aggregation play an essential role in the initial phase of thrombosis soon after an atherosclerotic plaque ruptures, breaks, or erodes. This process constitutes a significant pathogenic phase in the development of ACS-unstable angina (UA), STEMI, and NSTEMI. Antiplatelet therapies play an integral role in treatment in the setting of ACS since atherothrombotic events are fundamentally platelet-driven processes [7,8]. Therefore, it is essential to conduct studies to assess the efficacy and safety of antiplatelet therapy in ACS.
There are currently three groups of antiplatelet medications approved for treating and preventing ischemic events in ACS: cyclooxygenase (COX)-1 inhibitors such as aspirin; adenosine diphosphate (ADP) P2Y12 receptor antagonists like clopidogrel, prasugrel, ticagrelor, and cangrelor; and glycoprotein IIb/IIIa inhibitors (GPI) such as abciximab, eptifibatide, and tirofiban [8-11]. Aspirin, a COX inhibitor, was first used in cardiovascular prophylaxis in the 1980s, and the subsequent introduction of ADP receptor P2Y12 inhibitors not only provided an alternative for aspirin-intolerant patients but also the potential for high-intensity platelet inhibition due to concurrent blockade of both the COX and ADP-dependent pathways [12]. Aspirin acetylates an essential serine residue in the active region of the COX-1 isoenzyme, irreversibly inhibiting the COX activity of platelets and reducing thromboxane-dependent activation of platelets [12]. The platelet P2Y12 receptor binds ADP, activating the platelet glycoprotein IIb/IIIa receptor through Gi protein, increasing platelet degranulation, thromboxane generation, and aggregation of platelets [12]. Because of its importance in platelet activation, the P2Y12 receptor is a prominent target for antiplatelet drugs. Ticlopidine, a thienopyridine of the first generation, was developed in 1972 and approved as an antiplatelet agent in 1978. Despite its beneficial antiplatelet action, ticlopidine causes uncommon but possibly catastrophic adverse effects such as aplastic anemia, neutropenia, and thrombotic thrombocytopenic purpura, which call for constant hematological monitoring [13].
Clopidogrel, a second-generation thienopyridine with no hematological side effects of ticlopidine, was licensed for usage in 1997. Clopidogrel swiftly replaced ticlopidine due to its similar efficacy and better safety profile. Subsequently, other P2Y12 receptor blockers such as prasugrel and ticagrelor were introduced, which fix clopidogrel's disadvantages such as slow onset of action and variation among individuals in antiplatelet response [14]. Thienopyridine derivatives block the P2Y12 receptor irreversibly, resulting in antiplatelet action. Conversely, ticagrelor is a novel chemical family with a faster onset of effect with consistent inhibition of platelets that requires no hepatic metabolism and leads to reversible blockage of the P2Y12 receptor [15].
The last common pathway leading to platelet aggregation is mediated by fibrinogen binding to the glycoprotein IIb/IIIa receptor on the surface of platelets [16]. Glycoprotein IIb/IIIa receptor antagonists attach to the glycoprotein IIb/IIIa receptor, preventing fibrin from binding to the receptor [17]. Glycoprotein IIb/IIIa receptor inhibitors that have received clinical approval include abciximab, eptifibatide, and tirofiban. Their usage is typically not suggested for low- to moderate-risk individuals or patients with a conservative approach. In contrast, they provide the most significant benefit to those with severe ACS and undergoing percutaneous intervention (PCI) [18].
This review evaluates the available evidence regarding the various antiplatelets currently being used to treat ACS by addressing the following question: do the currently available antiplatelet agents benefit patients with ACS?
Rationale
Over the years, the use of antiplatelets in ACS has become increasingly prevalent, and hence being aware of the mode of action, safety, interactions with other medications, and adverse reactions of these widely used drugs will help guide future interventions, and this knowledge will serve as an excellent source of information for other medical professionals and researchers in addition to illuminating the gaps and current discrepancies in the understanding regarding the safety and efficacy of these drugs.
Objectives
This scoping review's foremost objective is to analyze various review articles to precisely (1) identify how the various antiplatelets work, including their uses, mechanism of action, drug interaction, and adverse effects; and (2) identify the efficacy and safety of antiplatelets in ACS, and determine if the antiplatelet agents currently in use benefit patients with ACS.
Review
Methods
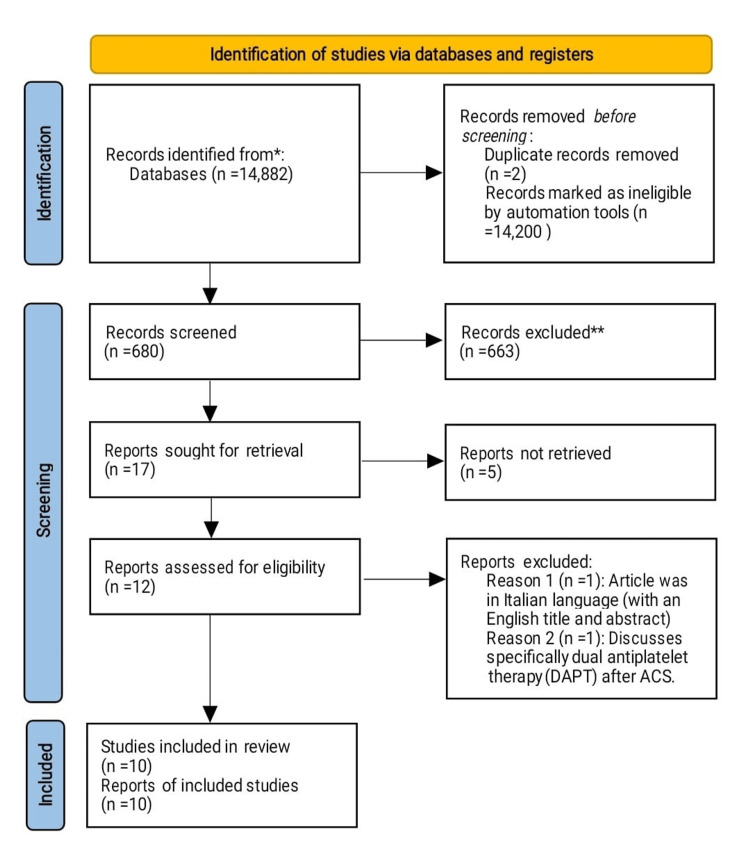
This scoping review focuses on clinical studies on the use of antiplatelet agents in ACS. We excluded animal studies and publications that only discussed the methodology related to antiplatelet agents without presenting clinical data. We adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19] 2020 (Figure 1) in conducting this review. We only used data collected from published papers, and hence ethical approval was not required.
Figure 1. PRISMA flow diagram illustrating the search strategy and study selection process.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ACS: acute coronary syndrome
Systematic Literature Search and Study Selection
A thorough search was conducted on the databases PubMed, Medline, and Google Scholar to look for pertinent papers. We looked for research cited in reviews, editorials, and commentaries on PubMed. We also diligently searched for further papers that met our inclusion criterion. We independently assessed a list of abstracts for inclusion based on specific criteria. The criteria included the efficacy and safety of antiplatelet drugs in ACS and a clearly described clinical cohort in the study. We excluded review papers and animal studies.
Inclusion and Exclusion Criteria
We established specific eligibility criteria for including and excluding papers to achieve our study goals. Our criteria are summarized in Table 1.
Table 1. The criteria adopted for the inclusion of studies.
| Inclusion criteria | Exclusion criteria |
| (a) Human studies | (a) Animal studies |
| (b) Published from 2013 to 2023 | (b) Methodological studies that only explain programming details |
| (c) English text | (c) Non-English texts |
| (d) Gender: all | (d) Age ≥45 years |
| (e) Age: 45+ years | (e) Studies involving clinical data other than cardiovascular disease |
| (f) Free papers | (f) Papers that needed to be purchased |
Search Strategy
A thorough literature review was conducted based on the population, intervention/ condition, control/comparison, and outcome (PICO) criteria. The search involved relevant keywords such as "antiplatelet therapy", "efficacy", "safety", and "acute coronary syndrome" on databases like PubMed (including Medline), and Google Scholar. The medical subject heading (MESH) approach for PubMed (including Medline) and Google Scholar, as detailed in Table 2, was employed to develop a comprehensive search strategy.
Table 2. The search strategy, search engines used, and the number of results elicited.
| Database | Search strategy | Results |
| PubMed | (((((Antiplatelet therapy[Title/Abstract]) OR (Efficacy of antiplatelet therapy[MeSH Terms])) OR (Safety of antiplatelet therapy[MeSH Terms])) OR (Antiplatelet on Acute Coronary Syndrome[MeSH Terms])) OR (Antiplatelet on Heart Attack[MeSH Terms])) AND (("2013/01/01"[Date - Publication] : "3000"[Date - Publication])) | 12,020 |
| PubMed (with filters) | (((((Antiplatelet therapy[Title/Abstract]) OR (Efficacy of antiplatelet therapy[MeSH Terms])) OR (Safety of antiplatelet therapy[MeSH Terms])) OR (Antiplatelet on Acute Coronary Syndrome[MeSH Terms])) OR (Antiplatelet on Heart Attack[MeSH Terms])) AND (("2013/01/01"[Date - Publication]: "3000"[Date - Publication])) AND ((y_10[Filter]) AND (ffrft[Filter]) AND (clinicaltrial[Filter] OR meta-analysis[Filter] OR randomizedcontrolledtrial[Filter] OR review[Filter] OR systematicreview[Filter])) Filters applied: Free full text, Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Review, Systematic Review, in the last 10 years, Humans, English, Female, Male, Middle Aged + Aged: 45+ years, MEDLINE | 582 |
| Google Scholar | (((Antiplatelet* OR Efficacy OR Safety) OR Acute Coronary Syndrome OR Heart Attack) AND Antiplatelet Therapy) Filters applied: Studies from 2013 to 2023, Review articles | 14,300 |
Quality Appraisal
We utilized various quality assessment tools to ensure the validity of the articles we shortlisted. We employed the PRISMA checklist and Cochrane risk-of-bias tool for randomized trials. Non-randomized clinical trials were evaluated using the Newcastle-Ottawa scale. We assessed the quality of qualitative studies, as shown in Table 3, by using the critical appraisal skills program (CASP) checklist.
Table 3. Quality appraisal tools used.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
| Tools used | Type of studies |
| Cochrane risk-of-bias tool | Randomized controlled trials (RCT) |
| Newcastle-Ottawa scale | Non-RCTs and observational studies |
| PRISMA checklist | Systematic reviews |
Results
We retrieved 14,882 articles from the three chosen databases PubMed, Medline, and Google Scholar. Following an in-depth analysis of each publication and the application of specific criteria, 14,202 papers were ultimately excluded, including two duplicate papers. We screened the remaining 680 papers and excluded 663 papers due to unsatisfactory titles and abstracts. From the remaining 17 papers, we filtered out another five papers because their content did not meet our inclusion standards. Of the 12 papers left, we excluded one written in the Italian language and another one that specifically discussed dual antiplatelet therapy (DAPT). The final step involved a rigorous quality review of the remaining 10 papers, all of which satisfied our standards. Our final review includes these 10 papers, which are summarized in Table 4.
Table 4. Summary of the findings of the selected papers.
ACS: acute coronary syndrome; PCI: percutaneous coronary intervention; DAPT: dual antiplatelet therapy; CAD: coronary artery disease
| Author/year | Country | Study design | Database used | Conclusion |
| Dash/2015 [20] | India | Review | PubMed | Antiplatelet therapy, like aspirin and clopidogrel, has a well-established therapeutic function in treating ACS, although recent research shows that prasugrel and ticagrelor have distinct advantages over clopidogrel. Numerous new antiplatelet drugs still need to be clinically validated to help establish the most efficient treatment regimen for patients with ACS or undergoing PCI |
| Franchi and Angiolillo/2014 [21] | USA | Review | PubMed and www.clinicaltrials.gov website | In several clinical trials, antiplatelet medication is crucial in managing and secondary prevention of ischemic episodes in individuals with ACS |
| Passacquale et al./2022 [12] | UK | Invited review | PubMed | Antiplatelet treatment is a vital component of cardiovascular prevention, provided the treatment is personalized for each patient based on a detailed evaluation of their thrombotic vs. bleeding risk |
| Kolandaivelu and Bhatt/2011 [22] | USA | Review | PubMed, Google Scholar, and Scopus | With proper medication adherence, antiplatelet therapy plays an essential role in preventing coronary heart disease |
| Lee/2015 [15] | Korea | Review | PubMed | Compared to aspirin and clopidogrel, aspirin and novel P2Y12 receptor blockers have a lower risk of cardiovascular complications following ACS. However, long-term DAPT raises the risk of severe bleeding, necessitating a careful balancing act between the risk of bleeding and anti-ischemic benefit |
| Packard et al./2012 [23] | USA | Review | Google Scholar, PubMed | In those suffering from CAD and ACS, dual antiplatelet treatment with aspirin plus clopidogrel or prasugrel has been proven efficient |
| Sharma et al./2020 [24] | India | Review | Google Scholar and PubMed | According to the most recent guidelines, patients with ACS are encouraged to use DAPT for 12 months. Nonetheless, as in any medical situation, an individual evaluation is necessary |
| Tendera and Wojakowski/2003 [25] | Poland | Review | PubMed, Google Scholar, and Scopus | Double antiplatelet therapy is more successful than aspirin alone in preventing stent thrombosis in coronary stenting |
| Udell et al./2015 [26] | Canada | Systematic review and meta-analysis | OVID Medline and Cochrane | DAPT treatment exceeding one year delivers a significant decrease in critical cardiovascular complications. It has to be considered in patients with past myocardial infarction who are at minimal bleeding risk |
| Tisdale/1998 [17] | USA | Journal article | PubMed | Patients with an acute previous myocardial infarction, history of stroke or prior transient ischemic attack, unstable angina, and various vascular illnesses experienced considerably fewer vascular-related complications due to the use of antiplatelet medication |
Discussion
Appropriate treatment with high efficacy and safety is necessary to enhance survival and outcome in patients with ACS. Based on the currently approved antiplatelet drugs, aspirin has been used as the core medication in patients with ACS. It acts by irreversibly blocking COX1, reducing the synthesis of thromboxane A2 (TXA2), which binds to thromboxane and prostaglandin endoperoxide (TP) receptors, and subsequently resulting in changes to platelet shape and increases platelet recruitment and aggregation [27]. As a result, aspirin reduces TP receptor-mediated platelet activation and aggregation [27]. When aspirin was first discovered, its use in ischemic heart diseases was debatable, apart from concerns regarding its side effects, such as peptic ulcer and gastrointestinal bleeding, especially when administered in large doses. Administering aspirin 1,000 mg/day could not prevent death in patients with prior myocardial infarction, according to the first major randomized study on the topic: the Aspirin Myocardial Infarction Study (AMIS) [28]. Moreover, adverse drug reactions were more prevalent in the aspirin group compared to the placebo group. However, a major, randomized study called the International Study of Infarct Survival (ISIS)-2 found that 160 mg/day of aspirin significantly reduced vascular mortality in patients with acute myocardial infarction by 23% compared to placebo [29]. The most widely used antiplatelet drug now is aspirin. For individuals with existing atherosclerotic coronary artery disease, current guidelines recommend using low-dose aspirin (75-325 mg/day) continuously [30].
The next group of antiplatelet drugs is called thienopyridines, P2Y12 inhibitors. An essential platelet agonist, ADP works by activating P2Y1 and P2Y12 receptors on the platelet plasma membrane. Of these two, the P2Y12 route exerts the most impact on platelet aggregation and stabilization [31]. Hence, blocking the action of the P2Y12 receptor exerts a great antiplatelet function. Ticlopidine, a first-generation drug, was discovered but had to be replaced with clopidogrel (second-generation) and the newer drugs prasugrel and ticagrelor due to the severe side effects [13] of ticlopidine. Cytochrome p450 isoforms first mediate the process of double oxidation of the prodrug clopidogrel in the liver before it becomes metabolically active. It selectively and irreversibly blocks the ADP P2Y12 receptor, ensuring that the effects remain for 7-10 days of platelet lifetime [32,33]. Given the fact that one group of antiplatelet agents can be so efficacious in treating and preventing cardiac ischemic events, how about combining two groups of drugs?
Dual antiplatelet therapy (DAPT) combines aspirin and a P2Y12 antagonist, namely clopidogrel. In individuals with ACS, DAPT has been proven to reduce recurrent major ischemic episodes [34]. The usual one-year prescription for DAPT is based on findings from significant early antiplatelet studies like CURE that analyzed the course of P2Y12 inhibition. Premature DAPT withdrawal has been associated with incidents of late stent thrombosis in patients with first-generation drug-eluting stents (DES). Hence, it was empirically pointed that DAPT be continued for up to 12 months in these individuals [35]. The CURE study identified that among 12,262 patients with NSTE-ACS, there was a significant decrease in the primary composite endpoint [cardiovascular (CV) death, non-fatal MI, or stroke] in the clopidogrel combined with aspirin arm versus aspirin alone at 12 months (9.3 vs. 11.4%, RR: 0.80, 95% CI: 0.72-0.90, p=0.001), with an increased risk of major bleeding (3.7 vs. 2.7%, RR: 1.38, p=0.001). Nevertheless, there was no substantial difference in mortality rates between treatment groups (5.1 vs. 5.5%, HR: 0.93, 95% CI: 0.79-1.08) [36]. Meanwhile, a meta-analysis by Udell et al. [26] stated that compared with aspirin alone, prolonged DAPT beyond one year produced a 22% relative and 1.1% absolute risk reduction for severe cardiovascular side effects throughout a mean 31-month follow-up involving more than 33,000 high-risk patients with stabilized MI. Therefore, it is recommended to use clopidogrel in combination with aspirin to treat ACS since it reduces long-term adverse events in patients with and without PCI [37].
Prasugrel, a third-generation thienopyridine, is an oral prodrug. In contrast to clopidogrel, prasugrel only needs a single oxidative step dependent on CYP to produce the active metabolite. Thus, prasugrel inhibits platelets more quickly, effectively, and consistently than clopidogrel because it is metabolized by esterases rather than CYP enzymes [38]. A phase 3 trial involving 13,608 patients [TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38)] showed that prasugrel (60 mg loading dose and a 10-mg daily maintenance dose) significantly decreased death, myocardial infarction, and stroke in patients with ACS who were scheduled for PCI compared to the recommended clopidogrel doses (300 mg loading dose and 75 mg per day), but with a higher risk of severe bleeding, including life-threatening hemorrhage [39-41]. However, prasugrel's net advantage compared to clopidogrel was particularly evident in individuals with STEMI [42].
The cyclopentyl-triazolopyrimidine class drug ticagrelor is a reversible P2Y12 antagonist that, in contrast to thienopyridines, is active on its own and is not dependent on hepatic metabolism to exert its pharmacological action. On the other hand, the drug undergoes extensive cytochrome-mediated oxidation, which results in the generation of an active by-product that also accounts for the beneficial effects [43]. Ticagrelor has a more rapid onset, as well as robust, and reliable antiplatelet activity than clopidogrel and a quicker offset of action [44].
Glycoprotein IIb/IIIa (GP IIb/IIIa) receptor inhibitors such as abciximab, eptifibatide, and tirofiban provide the most significant benefit only in those with severe ACS undergoing PCI and not for those who are at low- to moderate-risk [18], mainly not only due to its lack of beneficial effect but higher risk of mortality. There is a need for dose titration because high levels of platelet inhibition from oral GP IIb/IIIa receptor antagonists were linked to a very high incidence of major (11.8%) and minor (70%) bleeding events. This effect may result in long-lasting insufficient platelet inhibition, antagonist-induced prothrombotic activity, and a deficiency of protective properties [45,46]. However, most of the insufficient therapeutic advantages of oral GP IIb/IIIa receptor antagonists occurred with first-generation drugs like sibofiban, orbofiban, and xemilofiban may have been due to their distinct pharmacokinetics when they were previously administered for individuals with established IHD. Therefore, newly developed oral medications in this class, like roxifiban, were created and put through "response to dose" and "effectiveness" studies. Roxifiban is an oral non-peptide platelet GP IIb/IIIa receptor blocker that has excellent stability of plasma concentrations throughout dosage, a more potent blocking effect on tissue factor-induced platelet aggregation, higher affinity, longer elimination half-life (four to six days), and a more gradual breakdown from resting and activated platelets. These factors, taken together, could mitigate the side effects caused by the drug [47].
To sum up, all these classes of antiplatelet drugs evidently benefit those with IHD, especially ACS. While these agents are used to treat the condition, taking them to prevent the disease is also likely possible. As demonstrated by the PolyIran study, aspirin-containing polypill approaches have successfully hindered major cardiovascular events in low- and middle-income countries [48]. In contrast, Antithrombotic Trialists' collaboration in 2009 raised concerns about aspirin's net benefit in primary prevention due to an observed elevated chance of severe extracranial and gastrointestinal bleeding adverse effects despite having just a mild protection towards vascular events, as recorded in an extensive meta-analysis carried out by them [49]. Therefore, medical professionals must carefully assess the risk of bleeding in each patient and tailor the treatment accordingly to prevent unwanted complications.
Limitations
This systematic review has a few limitations. We included only human studies, specifically focused on those at least 45 years of age, and limited our research to English publications published in the past 10 years. Additionally, we only analyzed free articles and focused our research on English language papers regarding the efficacy and safety of antiplatelet therapy in ACS. Further studies are required to achieve more precise results and gain deeper insights into the topic.
Conclusions
In the rapidly evolving field of antiplatelet therapy, it could be a challenging prospect to thoroughly analyze and make sense of all the studies and research on the efficacy and safety of each antiplatelet medication, either as monotherapy or combination therapy in patients with ACS. As a growing number of novel medications are introduced, there could be a paradigm shift in how antiplatelet medications are administered to patients with ACS. Nevertheless, studies have successfully proven the favorable effects of antiplatelet treatment on ACS patients, with remarkable safety and efficacy, provided that the prescribed medication and dose have been customized individually, following a meticulous evaluation of the patient's risk of bleeding.
Acknowledgments
PBR, NU, and ND played a vital role in the conception and design of the data apart from editing corrections, tables, and figures. The article manuscript was drafted with assistance from NU, SD, and ND, and it was carefully reviewed for significant intellectual ideas by PBR, BP, and VT. SD and BP assisted with gathering and analyzing data. VT and PBR provided crucial help with data interpretation. BP took over the article submission and necessary follow-ups. Also, BP, PBR, VT, SD, ND, and NU carefully checked the whole paper to ensure any grammatical or literary issues were found and remedied. With deep gratitude, I acknowledge the efforts of each and every author of this article, who contributed significantly and profoundly to the conception, writing, editing, and revision of the text, and their combined efforts aided considerably in the preparation of the work. It is also important to emphasize that each author has given the final approval for the manuscript's publication, indicating their understanding and support of the data included. Moreover, they have willingly taken responsibility for the work's legitimacy and validity, showing they are committed to maintaining its scholarly integrity.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Tanusha Winson, Prithvi Basu Roy, Nanush Damarlapally, Nia Uswanti Binti Usman
Acquisition, analysis, or interpretation of data: Tanusha Winson, Prithvi Basu Roy, Vitrag N. Tejani, Sukhmeet S. Dhillon, Binay K. Panjiyar
Drafting of the manuscript: Tanusha Winson, Sukhmeet S. Dhillon, Nanush Damarlapally, Nia Uswanti Binti Usman
Critical review of the manuscript for important intellectual content: Tanusha Winson, Prithvi Basu Roy, Vitrag N. Tejani, Binay K. Panjiyar
Supervision: Tanusha Winson, Vitrag N. Tejani, Binay K. Panjiyar
References
- 1.Global perspective on acute coronary syndrome: a burden on the young and poor. Vedanthan R, Seligman B, Fuster V. Circ Res. 2014;114:1959–1975. doi: 10.1161/CIRCRESAHA.114.302782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardiovascular disease epidemiology in Asia: an overview. Ohira T, Iso H. Circ J. 2013;77:1646–1652. doi: 10.1253/circj.cj-13-0702. [DOI] [PubMed] [Google Scholar]
- 3.Inflammation, atherosclerosis, and coronary artery disease. Hansson GK. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Update on acute coronary syndromes: the pathologists' view. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Eur Heart J. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 5.Mechanisms of acute coronary syndromes and their implications for therapy. Libby P. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 6.In-hospital mortality of cardiogenic shock complicating ST-elevation myocardial infarction in Malaysia: a retrospective analysis of the Malaysian National Cardiovascular Database (NCVD) registry. Venkatason P, Zubairi YZ, Wan Ahmad WA, Hafidz MI, Ismail MD, Hadi MF, Zuhdi AS. BMJ Open. 2019;9:0. doi: 10.1136/bmjopen-2018-025734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Composition of coronary thrombus in acute myocardial infarction. Silvain J, Collet JP, Nagaswami C, et al. J Am Coll Cardiol. 2011;57:1359–1367. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Wright RS, Anderson JL, Adams CD, et al. Circulation. 2011;123:2022–2060. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- 9.2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Levine GN, Bates ER, Blankenship JC, et al. Circulation. 2011;124:0–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 10.ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation: the Task Force for the Management of Acute Coronary Syndromes (ACS) in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Hamm CW, Bassand JP, Agewall S, et al. Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 11.Guidelines on myocardial revascularization. Wijns W, Kolh P, Danchin N, et al. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 12.Antiplatelet therapy in cardiovascular disease: current status and future directions. Passacquale G, Sharma P, Perera D, Ferro A. Br J Clin Pharmacol. 2022;88:2686–2699. doi: 10.1111/bcp.15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ticlopidine and clopidogrel. Quinn MJ, Fitzgerald DJ. Circulation. 1999;100:1667–1672. doi: 10.1161/01.cir.100.15.1667. [DOI] [PubMed] [Google Scholar]
- 14.New P2Y(12) inhibitors. Cattaneo M. Circulation. 2010;121:171–179. doi: 10.1161/CIRCULATIONAHA.109.853069. [DOI] [PubMed] [Google Scholar]
- 15.Dual antiplatelet therapy for coronary artery disease. Lee CW. Circ J. 2015;79:255–262. doi: 10.1253/circj.CJ-14-1348. [DOI] [PubMed] [Google Scholar]
- 16.Platelet glycoprotein IIb/IIIa receptor inhibitors in ischemic heart disease. Lefkovits J, Topol EJ. Curr Opin Cardiol. 1995;10:420–426. doi: 10.1097/00001573-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Antiplatelet therapy in coronary artery disease: review and update of efficacy studies. Tisdale JE. Am J Health Syst Pharm. 1998;55:0–16. doi: 10.1093/ajhp/55.suppl_1.S8. [DOI] [PubMed] [Google Scholar]
- 18.Platelet glycoprotein IIb/IIIa inhibition in acute coronary syndromes. Gradient of benefit related to the revascularization strategy. Roffi M, Chew DP, Mukherjee D, et al. Eur Heart J. 2002;23:1441–1448. doi: 10.1053/euhj.2002.3160. [DOI] [PubMed] [Google Scholar]
- 19.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Liberati A, Altman DG, Tetzlaff J, et al. PLoS Med. 2009;6:0. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Current status of antiplatelet therapy in acute coronary syndrome. Dash D. Cardiovasc Hematol Agents Med Chem. 2015;13:40–49. doi: 10.2174/187152571301150730114514. [DOI] [PubMed] [Google Scholar]
- 21.Novel antiplatelet agents in acute coronary syndrome. Franchi F, Angiolillo DJ. Nat Rev Cardiol. 2015;12:30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 22.Antiplatelet therapy in coronary heart disease prevention. Kolandaivelu K, Bhatt DL. Cardiol Clin. 2011;29:71–85. doi: 10.1016/j.ccl.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Emerging antiplatelet therapy for coronary artery disease and acute coronary syndrome. Packard KA, Campbell JA, Knezevich JT, Davis EM. Pharmacotherapy. 2012;32:244–273. doi: 10.1002/j.1875-9114.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 24.Dual antiplatelet therapy in coronary artery disease. Sharma R, Kumar P, Prashanth SP, Belagali Y. Cardiol Ther. 2020;9:349–361. doi: 10.1007/s40119-020-00197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Role of antiplatelet drugs in the prevention of cardiovascular events. Tendera M, Wojakowski W. https://doi.org/10.1016/j.thromres.2003.08.003. Thromb Res. 2003;110:355–359. doi: 10.1016/j.thromres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Udell JA, Bonaca MP, Collet JP, et al. Eur Heart J. 2016;37:390–399. doi: 10.1093/eurheartj/ehv443. [DOI] [PubMed] [Google Scholar]
- 27.Low-dose aspirin for the prevention of atherothrombosis. Patrono C, García Rodríguez LA, Landolfi R, Baigent C. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 28.A randomized, controlled trial of aspirin in persons recovered from myocardial infarction. https://pubmed.ncbi.nlm.nih.gov/6985998/ JAMA. 1980;243:661–669. [PubMed] [Google Scholar]
- 29.Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. J Am Coll Cardiol. 1988;12:3–13. doi: 10.1016/0735-1097(88)92635-6. [DOI] [PubMed] [Google Scholar]
- 30.Cardiology patient page: aspirin. Paikin JS, Eikelboom JW. Circulation. 2012;125:0–42. doi: 10.1161/CIRCULATIONAHA.111.046243. [DOI] [PubMed] [Google Scholar]
- 31.Effects of P2Y(1) and P2Y(12) receptor antagonists on platelet aggregation induced by different agonists in human whole blood. Storey RF, Newby LJ, Heptinstall S. Platelets. 2001;12:443–447. doi: 10.1080/09537100120085450. [DOI] [PubMed] [Google Scholar]
- 32.Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. J Am Coll Cardiol. 2007;49:1505–1516. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 33.Clopidogrel response variability: current status and future directions. Ferreiro JL, Angiolillo DJ. Thromb Haemost. 2009;102:7–14. doi: 10.1160/TH09-03-0185. [DOI] [PubMed] [Google Scholar]
- 34.Dual antiplatelet therapy for secondary prevention of coronary artery disease. Degrauwe S, Pilgrim T, Aminian A, Noble S, Meier P, Iglesias JF. Open Heart. 2017;4:0. doi: 10.1136/openhrt-2017-000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Current trends in dual antiplatelet therapy: a 2017 update. Biasco L, Montrasio G, Moccetti M, Pedrazzini G. Cardiovasc Med. 2017;20:169–175. [Google Scholar]
- 36.PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. Full prescribing information. [ Nov; 2023 ];https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022307s007lbl.pdf 2012 11:2018. [Google Scholar]
- 37.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 38.Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Wallentin L, Varenhorst C, James S, et al. Eur Heart J. 2008;29:21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]
- 39.Prasugrel versus clopidogrel in patients with acute coronary syndromes. Wiviott SD, Braunwald E, McCabe CH, et al. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 40.Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. Antman EM, Wiviott SD, Murphy SA, et al. J Am Coll Cardiol. 2008;51:2028–2033. doi: 10.1016/j.jacc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Wiviott SD, Braunwald E, McCabe CH, et al. https://doi.org/10.1016/s0140-6736(08)60422-5. Lancet. 2008;371:1353–1363. doi: 10.1016/S0140-6736(08)60422-5. [DOI] [PubMed] [Google Scholar]
- 42.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM. Lancet. 2009;373:723–731. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 43.Metabolism of ticagrelor in patients with acute coronary syndromes. Adamski P, Buszko K, Sikora J, et al. Sci Rep. 2018;8:11746. doi: 10.1038/s41598-018-29619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Gurbel PA, Bliden KP, Butler K, et al. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 45.Platelet glycoprotein IIb/IIIa inhibitors: recognition of a two-edged sword? Quinn MJ, Plow EF, Topol EJ. Circulation. 2002;106:379–385. doi: 10.1161/01.cir.0000019581.22812.b2. [DOI] [PubMed] [Google Scholar]
- 46.Oral glycoprotein IIb/IIIa inhibitors: why don't they work? Chew DP, Bhatt DL, Topol EJ. Am J Cardiovasc Drugs. 2001;1:421–428. doi: 10.2165/00129784-200101060-00002. [DOI] [PubMed] [Google Scholar]
- 47.The use of roxifiban (DMP754), a novel oral platelet glycoprotein IIb/IIIa receptor inhibitor, in patients with stable coronary artery disease. Murphy J, Wright RS, Gussak I, et al. Am J Cardiovasc Drugs. 2003;3:101–112. doi: 10.2165/00129784-200303020-00004. [DOI] [PubMed] [Google Scholar]
- 48.Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Roshandel G, Khoshnia M, Poustchi H, et al. Lancet. 2019;394:672–683. doi: 10.1016/S0140-6736(19)31791-X. [DOI] [PubMed] [Google Scholar]
- 49.Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Baigent C, Blackwell L, Collins R, et al. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]