Abstract
It is of theoretical as well as practical interest to identify the components of the photosynthetic machinery that govern variability in photosynthesis rate (A) and water-use efficiency (WUE), and to define the extent by which the component processes limit A and WUE during developing water-deficit stress. For that purpose, leaf exchange of CO2 and H2O was determined in two growth-chamber-grown wheat cultivars (Triticum aestivum L. cv TAM W-101 and cv Sturdy), and the capacity of A was determined and broken down into carboxylation efficiency (c.e.), light- and CO2-saturated A, and stomatal conductance (gs) components. The limitations on A measured at ambient CO2 concentration (A350) were estimated. No cultivar difference was observed when A350 was plotted versus leaf water potential (Ψw). Light- and CO2-saturated A, c.e., and gs decreased with decreasing leaf Ψw, but of the corresponding photosynthesis limitations only those caused by insufficient c.e. and gs increased. Thus, reduced stomatal aperture and Calvin cycle activity, but not electron transport/photophosphorylation, appeared to be major reasons for drought stress-induced inhibition of A350. WUE measured as A350/gs first increased with stomatal closure down to a gs of about 0.25 mol H2O m−2 s−1 (Ψw = −1.6 MPa). However, it was predicted that A350/gs would decrease with more severe stress due to inhibition of c.e.
Full text
PDF
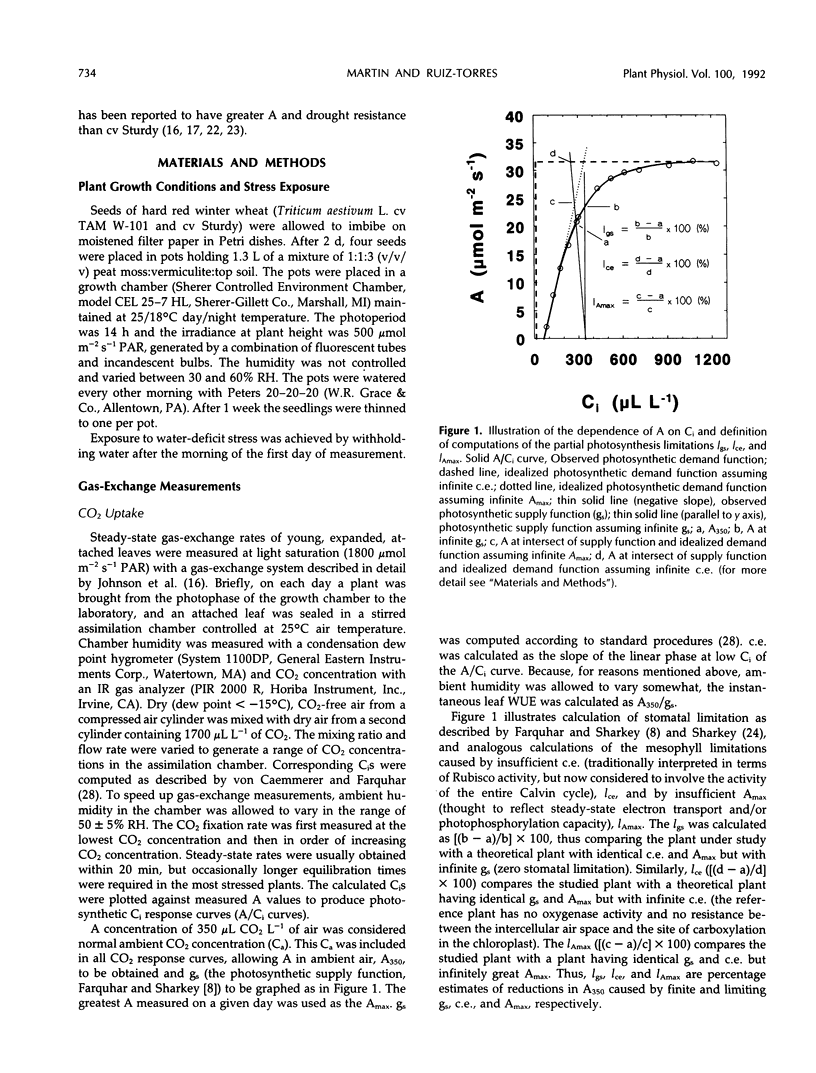
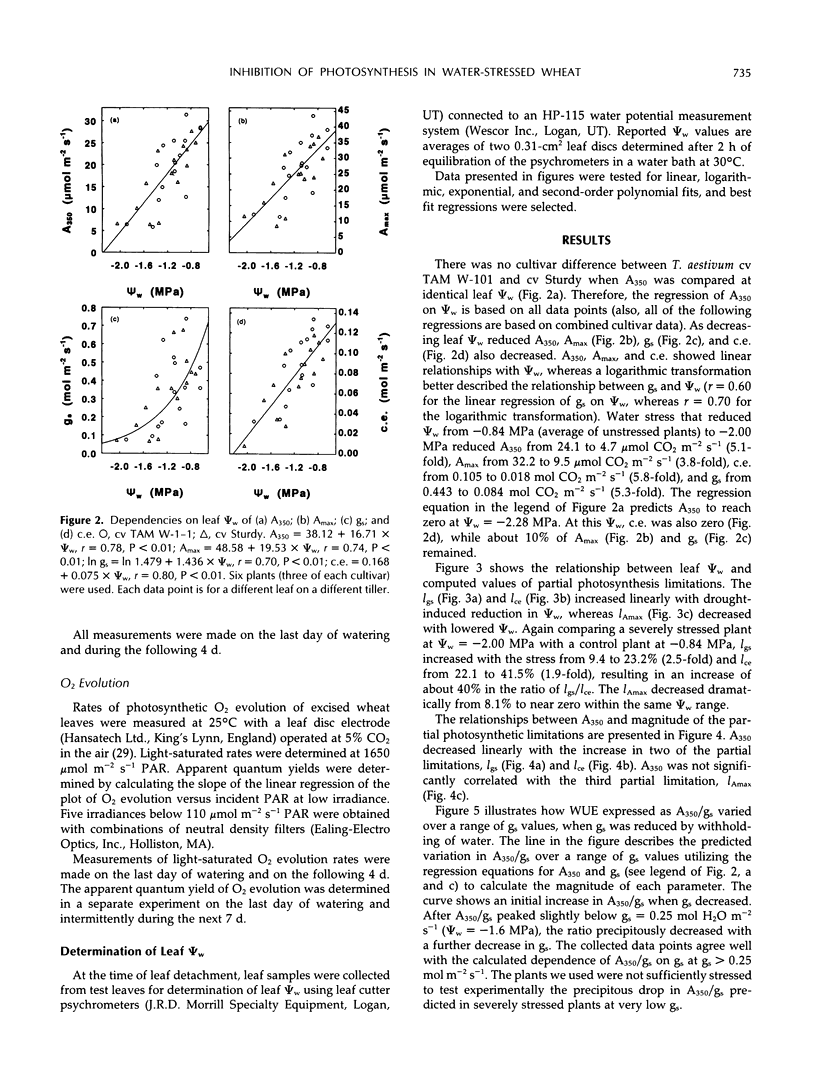
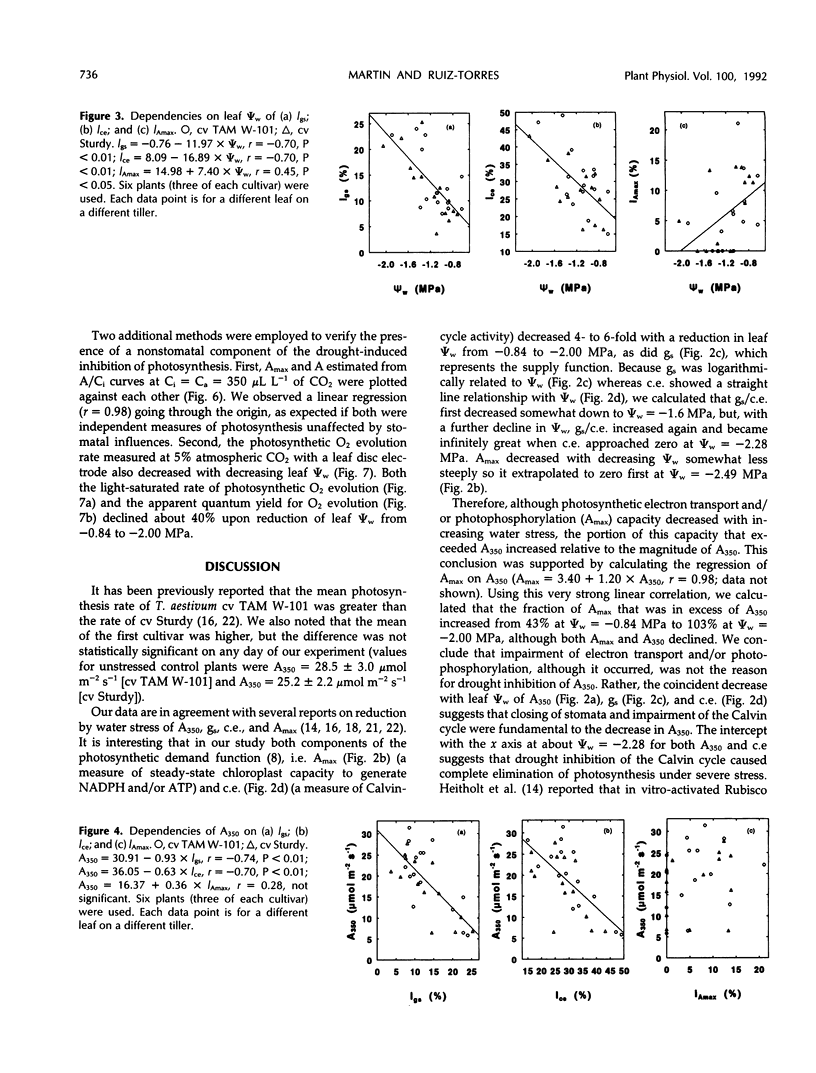
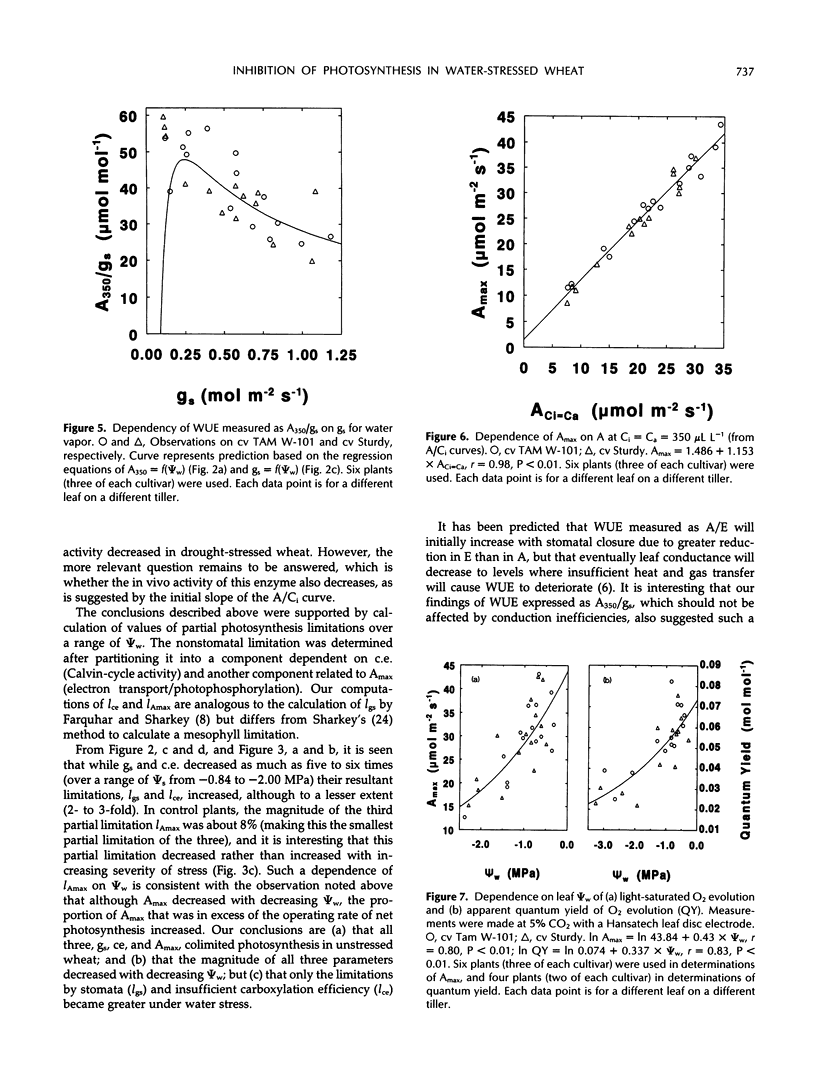


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. S. Plant productivity and environment. Science. 1982 Oct 29;218(4571):443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Keck R. W., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: III. Differing Inhibition of Electron Transport and Photophosphorylation. Plant Physiol. 1974 Mar;53(3):474–479. doi: 10.1104/pp.53.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Nienhuis J., King G., Schaefer A. Restriction fragment length polymorphisms associated with water use efficiency in tomato. Science. 1989 Mar 31;243(4899):1725–1728. doi: 10.1126/science.243.4899.1725. [DOI] [PubMed] [Google Scholar]
- Martin B., Thorstenson Y. R. Stable Carbon Isotope Composition (deltaC), Water Use Efficiency, and Biomass Productivity of Lycopersicon esculentum, Lycopersicon pennellii, and the F(1) Hybrid. Plant Physiol. 1988 Sep;88(1):213–217. doi: 10.1104/pp.88.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. A., Boyer J. S. Acclimation of photosynthesis to low leaf water potentials. Plant Physiol. 1984 Jan;74(1):161–166. doi: 10.1104/pp.74.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassey T. L., Sharkey T. D. Mild Water Stress of Phaseolus vulgaris Plants Leads to Reduced Starch Synthesis and Extractable Sucrose Phosphate Synthase Activity. Plant Physiol. 1989 Apr;89(4):1066–1070. doi: 10.1104/pp.89.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]


