Significance
Coral reefs are in jeopardy as climate change leads to increasingly frequent marine heatwaves. Some corals survive these extreme events, and this exposure may prime corals to increase their heat tolerance. Yet, as the time between heatwaves decreases, the accumulation of stress experienced may preclude opportunities for beneficial gains in heat tolerance. This nearly decade-long study revealed that repetitive exposure to heatwaves leads to divergent coral bleaching and recovery trajectories. Some corals increased bleaching resistance and demonstrated physiological recovery, whereas others exhibited alarming seasonal bleaching and accumulating mortality without heat stress following successive heatwaves. As the climate continues to change, surviving corals must not only gain heat tolerance but also rapidly recover to maintain critical ecosystem services that humans rely on.
Keywords: climate change, ocean warming, coral bleaching, environmental memory, acclimatization
Abstract
Increasingly frequent marine heatwaves are devastating coral reefs. Corals that survive these extreme events must rapidly recover if they are to withstand subsequent events, and long-term survival in the face of rising ocean temperatures may hinge on recovery capacity and acclimatory gains in heat tolerance over an individual’s lifespan. To better understand coral recovery trajectories in the face of successive marine heatwaves, we monitored the responses of bleaching-susceptible and bleaching-resistant individuals of two dominant coral species in Hawai’i, Montipora capitata and Porites compressa, over a decade that included three marine heatwaves. Bleaching-susceptible colonies of P. compressa exhibited beneficial acclimatization to heat stress (i.e., less bleaching) following repeat heatwaves, becoming indistinguishable from bleaching-resistant conspecifics during the third heatwave. In contrast, bleaching-susceptible M. capitata repeatedly bleached during all successive heatwaves and exhibited seasonal bleaching and substantial mortality for up to 3 y following the third heatwave. Encouragingly, bleaching-resistant individuals of both species remained pigmented across the entire time series; however, pigmentation did not necessarily indicate physiological resilience. Specifically, M. capitata displayed incremental yet only partial recovery of symbiont density and tissue biomass across both bleaching phenotypes up to 35 mo following the third heatwave as well as considerable partial mortality. Conversely, P. compressa appeared to recover across most physiological metrics within 2 y and experienced little to no mortality. Ultimately, these results indicate that even some visually robust, bleaching-resistant corals can carry the cost of recurring heatwaves over multiple years, leading to divergent recovery trajectories that may erode coral reef resilience in the Anthropocene.
Ocean warming driven by climate change has led to staggering losses of live coral on coral reefs worldwide and is among the most pressing of stressors threatening the survival of coral reefs today (1, 2). As mean ocean surface temperatures have steadily increased, there has been a corresponding increase in the occurrence of marine heatwaves (3, 4). These extreme warm water events can persist for days to months, frequently leading to coral bleaching, a symptom of the breakdown of the symbiosis between corals and their dinoflagellate algal endosymbionts (family Symbiodiniaceae) (5). This symbiosis is the energetic foundation of coral reef ecosystems, allowing for high rates of productivity in otherwise oligotrophic seas (6). The energy corals receive from symbiont photosynthesis supports a majority of their metabolic demand (7), thus fueling the construction of the complex three-dimensional framework necessary to support the most biodiverse ecosystems in the ocean (8). Given this significance, the breakdown of the coral–algal symbiosis during coral bleaching can have devastating consequences for coral reef ecosystems, ranging from declines in primary production to widespread coral mortality and reef erosion (1, 9). These losses can lead to concomitant declines in biodiversity and ecosystem function that harm not just these ecosystems but also the human societies that rely on the myriad services functioning coral reefs provide (10). As marine heatwaves become increasingly frequent and severe (11), coral bleaching events are predicted to correspondingly increase (12). However, the extent to which individual corals can acclimatize to these conditions within their lifetime and thus gain resistance to recurring marine heatwaves remains a critical outstanding question.
Environmental memory of thermal stress, defined as the retention of information from an initial exposure that modifies the response to a later exposure, has been posited to lead to beneficial acclimatization of individuals exposed to repeat marine heatwaves (13–16). Indeed, coral communities that experienced sublethal heat stress and/or bleaching during marine heatwaves were less prone to bleaching in subsequent exposures across the Caribbean (17, 18), Great Barrier Reef (19), and Indo-Pacific (15, 16, 20, 21). There are likely multiple factors driving these increases in coral community heat tolerance, including beneficial acclimatization of surviving individuals (e.g., epigenetic modifications) (13–15), shifts in community composition driven by losses of bleaching-susceptible individuals (19, 22–25), and selection for thermally tolerant offspring (i.e., adaptation) (26). However, exposure to sublethal heat stress in individual corals may not always lead to benefits in subsequent exposures (27), as a history of heat stress could exacerbate the effects of subsequent heat stress (i.e., sensitization) when corals have not fully recovered (28–30). In light of the increasing frequency and severity of marine heatwaves (11), there is a growing need to better understand how individual corals are responding to and recovering from repeat heatwaves and the ecological consequences of environmental memory for reef resilience into the future. Encouragingly, phenotypic diversity in bleaching susceptibility varies both within and between coral species, which is likely an important source of adaptive variation (31) that could lead to directional selection of bleaching resistance (e.g., ref. 20). However, the pace of ocean warming requires rapid acclimatization of established adult colonies in order to buy time for adaptation and proliferation of the next generation of thermally tolerant corals, which may take several decades to centuries in these long-lived and slow-growing species (32). As marine heatwaves have only recently begun occurring on multidecadal time scales (1), we are just beginning to understand the capacity for individuals to recover, acclimatize, or sensitize following heat stress (14). Further, understanding how phenotypic variation in these responses between individuals and species influences population dynamics in the field remains underexplored, yet has important implications for predicting ecosystem diversity and function in the face of a rapidly changing climate (5).
In order to address this critical gap in our knowledge, we examined the responses of coral communities and individual colonies of two dominant reef-building coral species in Hawai’i, Montipora capitata and Porites compressa, throughout a decade that included three marine heatwaves (2014, 2015, and 2019). Adjacent conspecific colonies with contrasting bleaching phenotypes (i.e., bleaching-resistant vs. bleaching-susceptible) were first identified in 2015 during the second of two consecutive coral bleaching events that occurred in Kāne‘ohe Bay in 2014 and 2015 (33). Individual coral pigmentation, cumulative colony mortality, and reef-wide bleaching prevalence were tracked across the next 9 y (2015 to 2023), which included another bleaching event in 2019 (34). Following the 2019 event, we repeatedly assessed a suite of physiological parameters (e.g., metabolic rates, symbiont densities, host biomass and tissue composition) over 3 y to better understand intra- and interspecific variation in individual colony recovery trajectories following repeat heat stress. In addition, we explored the capacity for environmental memory to influence coral thermotolerance through a standardized short-term heat stress assay of these same individuals (35). This nearly decade-long investigation of individual coral colonies that encompass the phenotypic extremes within populations of these two species in the field is a powerful framework to test the hypothesis that divergent responses to repeat in situ heat stress lead to variation in recovery trajectories, with consequences for future heat tolerance and coral reef survival.
Results
Three Successive Heatwaves Influenced Reef-Wide Coral Bleaching Prevalence.
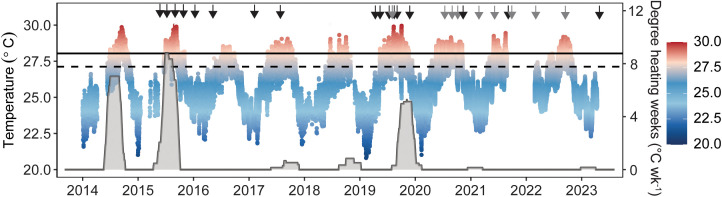
Between 2014 and 2023, three significant thermal anomalies were documented across Kāne‘ohe Bay (Fig. 1 and SI Appendix, Figs. S1 and S2 and Tables S1 and S2). During these events, maximum sustained degree heating weeks (DHW) at Patch Reef 13 (PR13) were lower in 2014 (7.3 °C wk−1) than 2015 (8.8 °C wk−1) and lowest in 2019 (5.1 °C wk−1) (Fig. 1 and SI Appendix, Tables S1 and S2). In contrast, 2019 was the most severe of the three events in the southern end of the bay and regionally (10 to 14 °C wk−1; SI Appendix, Table S2). From 2015 to 2023, coral cover at PR13 was dominated by P. compressa (53.5% ± 0.9) and M. capitata (12.5% ± 0.5) (SI Appendix, Fig. S3). At the peak of the 2015 heatwave, reef-wide bleaching prevalence reached 39.1% for M. capitata and 44.9% P. compressa, declining 2 mo after the heatwave in P. compressa (1.4%), but not M. capitata (35.5%) (SI Appendix, Fig. S4). The following summer, bleaching prevalence across PR13 reached 38.1% for M. capitata in the absence of any measurable heat stress but was rarely observed for P. compressa (0.2%) (Fig. 2). This cyclic seasonal pattern of decreased bleaching prevalence of M. capitata in winter and increased bleaching prevalence in summer continued the following year. At the peak of the 2019 heatwave, reef-wide bleaching prevalence increased to similar levels observed in the 2015 heatwave in both M. capitata (45.9%) and P. compressa (38.8%) (SI Appendix, Fig. S4); however, despite similar prevalence, the severity of bleaching was threefold lower for P. compressa in 2019 than 2015 (34). Notably, bleaching prevalence again showed a cyclic seasonal pattern in M. capitata, declining in winter and increasing in each of the three summers following 2019 (September 2020: 46.9%; October 2021: 21.7%; September 2022: 14.9%). In contrast, reef-wide bleaching prevalence of P. compressa did not change between seasons and remained low (0.7 to 3.2%) (SI Appendix, Fig. S4).
Fig. 1.
Temperature profile and heat stress accumulation over time. In situ temperatures were recorded from January 2014 to April 2023 at a depth of 0.7 to 2.7 m in Kāne‘ohe Bay. Points indicate hourly measurements (metadata in SI Appendix, Table S1). Heat stress accumulation was estimated by DHW (gray shading) calculated from mean daily (24 h) temperatures. Black arrows indicate image collection, and gray arrows indicate image collection with physiological sampling. The dashed horizontal line indicates the Kāne‘ohe Bay’s climatological maximum monthly mean (MMM; 27.3 °C), and the solid horizontal line indicates the local coral bleaching threshold (MMM + 1 °C; 28.3 °C).
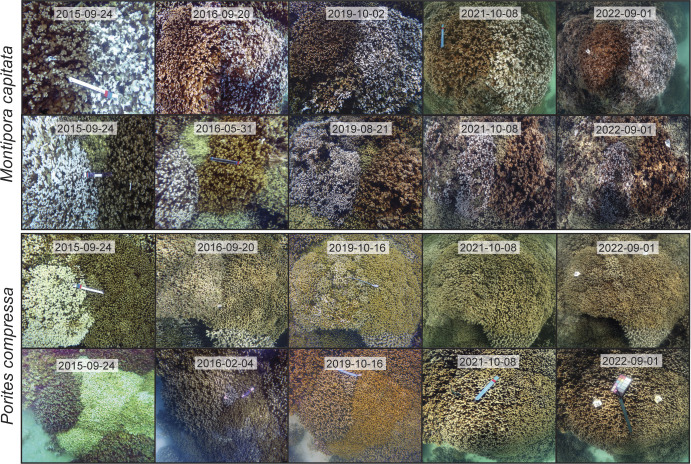
Fig. 2.
Representative images of M. capitata and P. compressa pairs over time in Kāne‘ohe Bay. Each row depicts a single pair of bleaching-susceptible and bleaching-resistant individuals from 2015 to 2022 (the Inset indicates the date the image was taken), and the corals appear in the same orientation in each image within each row.
Acclimatization and Sensitization in Colony Phenotypes across Recurring Heatwaves.
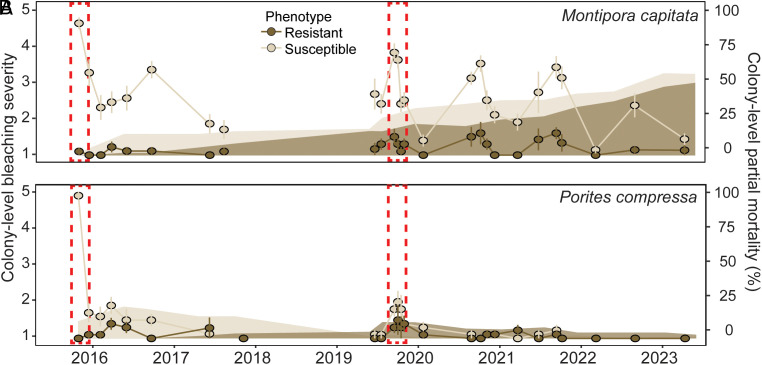
Colony-level bleaching severity was tracked across the same individuals (SI Appendix, Tables S3 and S4 and Figs. S5 and S6) and was influenced by the significant four-way interaction between coral species, phenotype, season (i.e., winter vs. summer), and time (F = 6.12, P < 0.0001) (SI Appendix, Table S5). During the 2015 heatwave, bleaching-susceptible M. capitata and P. compressa underwent the greatest bleaching (e.g., 100% bleached), whereas bleaching-resistant conspecifics remained fully pigmented (P < 0.0001) (Figs. 2 and 3). Throughout the next 2 y (2016 to 2017), bleaching-susceptible M. capitata remained half as pigmented as bleaching-resistant conspecifics regardless of season (P ≤ 0.04) (Figs. 2 and 3). Interestingly, P. compressa phenotypes were indistinguishable by the summer after the heatwave (P = 0.23), and remained so throughout 2017 (Figs. 2 and 3). During the 2019 marine heatwave, bleaching-susceptible P. compressa (P < 0.03) and bleaching-susceptible M. capitata (P < 0.0001) were again less pigmented than bleaching-resistant conspecifics. Compared to the previous 2015 heatwave when all susceptible individuals were severely bleached, bleaching-susceptible M. capitata were moderately bleached (30% less severe) during the 2019 heatwave, whereas bleaching-susceptible P. compressa were only mildly affected (Figs. 2 and 3). For the next 2 y (2020 to 2021), bleaching-susceptible M. capitata remained half as pigmented than bleaching-resistant conspecifics regardless of season (P < 0.0001), whereas P. compressa phenotypes were indistinguishable by winter 2020 and remained so throughout 2021 to 2023 (P > 0.49) (Figs. 2 and 3). By winter 2022, M. capitata phenotypes were indistinguishable for the first time during the study period following the initial bleaching (P = 0.5), yet in the subsequent summer, bleaching-susceptible M. capitata were again less pigmented than bleaching-resistant phenotypes (P < 0.0001) (Figs. 2 and 3).
Fig. 3.
Bleaching and mortality response of individual bleaching-resistant and bleaching-susceptible corals over time. Colony-level bleaching severity (solid lines; mean ± SE) and cumulative colony-level partial morality (shading; mean) from September 2015 to April 2023 for individual colonies of (A) M. capitata (n = 9 to 10) and (B) P. compressa (n = 7 to 10). Colony-level bleaching severity was visually determined for each colony following the methodology of ref. 34, where (1) represents 0% bleached and (5) >80% bleached. Dashed red rectangles indicate the 2015 and 2019 marine heatwaves.
Colony-level partial mortality was influenced by the significant interaction between species and time (χ2 = 86.9, P < 0.0001) (SI Appendix, Table S6). Following the 2015 marine heatwave, there were no detectable differences in partial mortality between M. capitata and P. compressa (P > 0.42) (Figs. 2 and 3); however, bleaching-susceptible individuals of both M. capitata (14%) and P. compressa (21%) exhibited significantly greater partial mortality than bleaching-resistant conspecifics (0% for M. capitata, 2% for P. compressa) in the first 2 y following that heatwave (Figs. 2 and 3; 33). In the first 6 mo following the 2019 heatwave, M. capitata exhibited significantly greater partial mortality across both bleaching-susceptible (20%) and bleaching-resistant (31%) individuals than P. compressa (<10% for both phenotypes) (P < 0.03) (Figs. 2 and 3). In the 3 y after the 2019 heatwave (2020 to 2023), M. capitata partial mortality steadily increased across both bleaching-susceptible (up to 56%) and bleaching-resistant (up to 47%) individuals, while P. compressa remained low (<5%). From the 2019 heatwave onward, there were no significant differences of phenotype, interactive or otherwise (χ2 = 20.7, P = 0.11).
Intra- and Interspecific Trajectories in Physiological Metrics Indicate Prolonged and Incomplete Recovery.
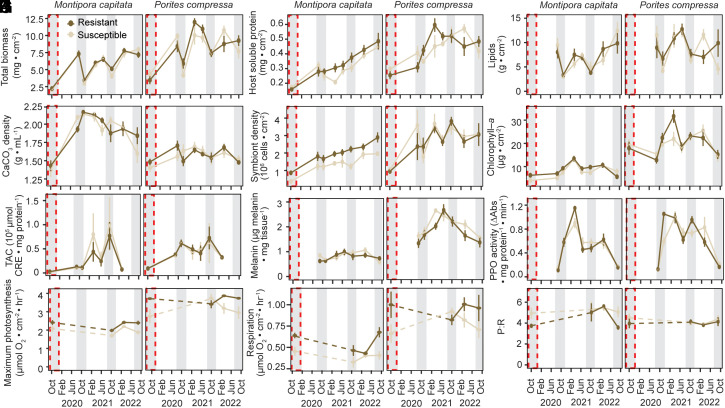
Coral physiological parameters were assessed from October 2019 (during the marine heatwave) to September 2022 (35 mo post heat stress) (Fig. 4 and SI Appendix, Fig. S7 and Table S7). Host tissue biomass (χ2 = 15.9, P < 0.03) and host lipid densities (χ2 = 25.2, P < 0.0003) were significantly influenced by the interaction between coral species and time (Fig. 4) (SI Appendix, Table S8). Specifically, P. compressa had 20 to 90% greater host tissue biomass than M. capitata across the time series (P < 0.003), with biomass converging 3 y after the 2019 marine heatwave (P = 0.24) (Fig. 4A and SI Appendix, Fig. S7). For both M. capitata and P. compressa, tissue biomass and lipid densities displayed strong seasonality, where tissue parameters were greatest March–June prior to annual coral spawning (Fig. 4). During seasonal temperature maxima, tissue biomass for both coral species did not differ from the 2019 marine heatwave in the first summer following the heatwave (2020, P > 0.44), although biomass eventually increased by 95% in the second summer (2021, P < 0.0008) and 163% by the third summer (2022, P < 0.0001) (SI Appendix, Fig. S7).
Fig. 4.
Physiological response of bleaching-resistant and bleaching-susceptible corals over time. (A) Host total tissue biomass (ash-free dry weight), (B) host soluble protein density, (C) host lipid density, (D) calcium carbonate (CaCO3) density, (E) endosymbiont cell density, (F) chlorophyll-a concentration, (G) host total antioxidant capacity (TAC), (H) host melanin content, (I) host prophenoloxidase (PPO) activity, (J) maximum photosynthetic rates, (K) light-enhanced dark respiration rates, and (L) photosynthesis to respiration ratios (P:R) for bleaching-susceptible and bleaching-resistant M. capitata and P. compressa. Points and error represent the mean ± SE (n = 4 to 10). The gray shading indicates seasonal peak temperatures (i.e., September–October), with a dashed red rectangle representing the 2019 marine heatwave.
Host soluble protein density was significantly influenced by the interaction between coral species and time (χ2 = 20.5, P < 0.004) (SI Appendix, Table S8). Generally, P. compressa tissues had ~40% greater protein concentration than M. capitata across the time series (P < 0.008), apart from 10 mo post heat stress (P = 0.1), and later with protein concentration of the two species converging 3 y after the 2019 marine heatwave (2022; P = 0.55) (Fig. 4B). For M. capitata, protein concentration showed a stepwise increase over time, with concentration significantly greater across all time points when compared to the 2019 marine heatwave (P < 0.05). While bleaching-susceptible M. capitata protein concentration trended lower than bleaching-resistant conspecifics, there was no significant influence of phenotype (χ2 = 0.11, P = 0.74). For P. compressa, there was no detectable difference in protein concentration between the 2019 marine heatwave and 10 mo post heat stress (P = 0.16), yet by 13 mo, protein concentration was significantly greater (40 to 89%) across all subsequent time points (P < 0.01) (Fig. 4B and SI Appendix, Fig. S7). Interestingly, during seasonal temperature maxima, P. compressa protein concentration was 35% lower 13 mo post heat stress than 24 mo (P = 0.003), yet there were no observable differences between 24 and 35 mo (P = 0.46) (SI Appendix, Fig. S7).
Calcium carbonate (CaCO3) bulk density was significantly influenced by the three-way interaction between coral species, phenotype, and time (χ2 = 14.1, P = 0.05) (SI Appendix, Table S6). During the 2019 marine heatwave, CaCO3 density declined by up to 50% for M. capitata and up to 14% for P. compressa regardless of phenotype, at which time density was indistinguishable between coral species (P > 0.72) (Fig. 4D and SI Appendix, Fig. S7). Across the rest of the time series, CaCO3 density was nearly 20% greater in M. capitata when compared to P. compressa regardless of phenotype (P < 0.05), with the exception of bleaching-susceptible corals at 35 mo post heat stress (P = 0.52). For bleaching-resistant M. capitata, however, CaCO3 density was up to 55% greater across all time points when compared to the 2019 heatwave (P < 0.002) (Fig. 4). For bleaching-susceptible M. capitata, CaCO3 density was also significantly greater across all time points when compared to the 2019 heatwave (32 to 47%) (P < 0.0001), with the exception of 35 mo postheat stress when CaCO3 density was depressed (5% lower than 29 mo post heat stress) (P = 0.59) (Fig. 4D). Although bleaching-susceptible P. compressa trended lower than bleaching-resistant conspecifics during and 10 mo after the marine heatwave, pairwise comparisons revealed that, for P. compressa of either phenotype, CaCO3 density was not significantly different across time (P > 0.05) (Fig. 4D and SI Appendix, Fig. S7).
Symbiont densities were significantly influenced by the interaction between coral species and time (χ2 = 16.4, P = 0.02) (SI Appendix, Table S8). While bleaching-susceptible M. capitata symbiont densities were approximately 50% lower than bleaching-resistant conspecifics across the study, the influence of phenotype was not statistically significant (χ2 = 1.15, P = 0.28) (Fig. 4E and SI Appendix, Fig. S7). Pairwise comparisons revealed that, generally, P. compressa had ~60% greater symbiont densities than M. capitata, apart from during the 2019 heatwave when both species experienced declines (P = 0.25), and at 29 and 35 mo post heat stress (P > 0.07), when symbiont densities were greatest (Fig. 4E and SI Appendix, Fig. S7). For M. capitata, symbiont densities did not rebound immediately after the 2019 heatwave, and were indistinguishable from the 2019 heatwave until 17 mo post heat stress, after which symbiont densities increased by 200% by 2023. In contrast, P. compressa symbiont densities were significantly greater across all time points when compared to the 2019 marine heatwave (~175 to 260%) (P < 0.02) and indicated seasonal patterns (Fig. 4E). For P. compressa, symbiont densities were significantly higher 24 mo post heat stress than 13 mo (P = 0.0005), yet there was no significant difference between 24 and 35 mo (P = 0.74) (SI Appendix, Fig. S7).
Chlorophyll-a content was significantly influenced by the three-way interaction between coral species, phenotype, and time (χ2 = 14.9, P = 0.04) (SI Appendix, Table S8). Pairwise comparison revealed only one significant difference between bleaching-susceptible or bleaching-resistant phenotypes; that is, in March 2021, bleaching-susceptible P. compressa had 50% lower chlorophyll-a content than bleaching-resistant conspecifics (Fig. 4F). The individual effects of time (χ2 = 24.67, P < 0.0008) and coral species (χ2 = 46.8, P < 0.0001) also emerged as significant, where P. compressa had significantly greater chlorophyll-a content than M. capitata (up to 130%). Post hoc analyses revealed for both species chlorophyll-a content did not differ from the 2019 marine heatwave until 17 mo postheat stress (March 2021) (P < 0.0001), when chlorophyll-a was greatest, yet, in June 2021 and September 2022, chlorophyll-a declined to values indistinguishable from the heatwave (P > 0.91) (Fig. 4F and SI Appendix, Fig. S7). Metabolic rates, immunity [i.e., melanin content, prophenoloxidase (PPO)] and total antioxidant capacity (TAC) were influenced by the interaction of coral species and time, with all significant patterns detailed in Fig. 4 G–L and SI Appendix, Fig S7.
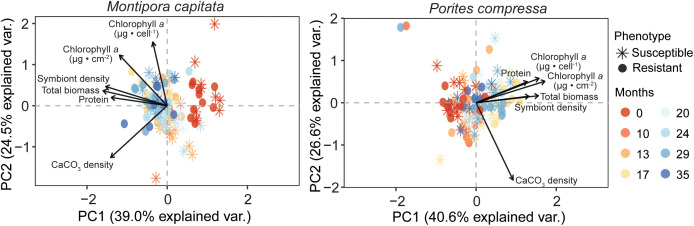
Multivariate Physiology Reveals Divergent Responses across Species and Time.
For both M. capitata and P. compressa, permutational multivariate ANOVA (PERMANOVA) of physiological traits revealed a significant effect of time (P <0.0001) (SI Appendix, Table S9). Pairwise comparisons revealed that multivariate physiology for all time points were significantly different from the marine heatwave for both species (P < 0.005) (Fig. 5). For M. capitata, all other comparisons were significantly different, apart from November 2020 and October 2021 (P = 0.11) and June 2021 and October 2021 (P = 0.21). For P. compressa after >1 y recovery from the marine heatwave, multivariate physiology indicated seasonal patterns, with no significant differences uncovered between August 2020 and September 2022 (P = 0.26), November 2020 and October 2021 (P = 0.16), and March 2021 and March 2022 (P = 0.62).
Fig. 5.
Principal component analysis (PCA) of physiological traits of individual bleaching-resistant and bleaching-susceptible corals over time. Biplot vectors represent physiological traits (solid arrows), and points indicate the multivariate physiology of each coral genet (n = 4 to 10). Colors of the points indicate the number of months postheat stress, where “0” (red) represents the 2019 marine heatwave.
Standardized Acute Heat Stress Assays Confirm That Environmental Memory Influences Heat Tolerance.
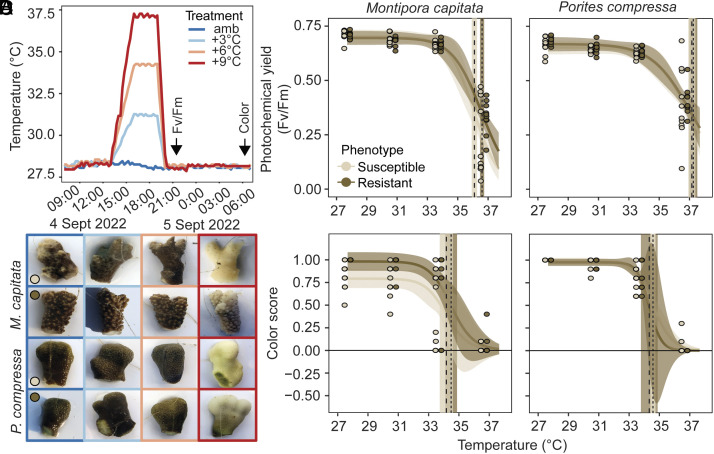
Photochemical yield (Fv/Fm) was significantly influenced by the interaction between treatment and species (Χ2 = 35.3, P < 0.0001) as well as treatment and phenotype (Χ2 = 9.1, P = 0.03) (SI Appendix, Table S10). For both species, significant declines in photochemical yield were only observed in the most extreme treatment (P < 0.0001), declining by over 50% in most individuals (Fig. 6). Further, regardless of species, significantly lower photochemical yield was found in bleaching-susceptible compared to bleaching-resistant corals at +9 °C (P = 0.01), whereas no detectable differences in phenotype were found between the three other treatments (P > 0.74). There was a 1.18 °C range in ED50 (effective dose 50) between the two species, with P. compressa more heat tolerant than M. capitata. Bleaching-susceptible M. capitata were the least heat tolerant, with a 50% reduction in Fv/Fm observed at 36.1 °C (95% CI: 35.8 to 36.5 °C) vs. 36.6 °C (95% CI: 36.3 to 36.9 °C) for bleaching-resistant conspecifics (Fig. 6C). For P. compressa, bleaching-susceptible corals were virtually indistinguishable from bleaching-resistant phenotypes, with a 50% reduction in Fv/Fm observed at 37.2 °C (95% CI: 36.7 to 37.6 °C) and 37.3 °C (95% CI: 36.8 to 37.8 °C), respectively. Color scores revealed similar patterns, with bleaching-susceptible M. capitata exhibiting lower ED50 (34.2 °C; 95% CI: 33.3 to 35.1 °C) than bleaching-resistant conspecifics (34.5 °C; 95% CI: 33.7 to 35.3 °C) (Fig. 6D). For P. compressa, color score ED50 was indistinguishable between bleaching-resistant and bleaching-susceptible P. compressa phenotypes (34.4 °C and 34.6 °C, respectively) (Fig. 6D).
Fig. 6.
Variation in heat tolerance across bleaching-susceptible and bleaching-resistant corals from experimental heat stress assay. (A) Temperature measurements (recorded every 15 min) of the controlled experimental heat stress assays (amb = ambient temperature of 27.7 °C), with an arrow indicating when dark-adapted photochemical yield (Fv/Fm) and color score were determined. (B) Respective images of coral fragments during the short-term heat stress. Three-parameter log-logistic dose–response curves were fitted to (C) Fv/Fm measurements (±95% CI) and (D) color score measurements (±95% CI) in response to temperature for M. capitata and P. compressa, where points indicate individual measures for coral genets (n = 10) in each treatment. Dashed vertical lines indicate the mean ED50 for each phenotype (dashed = susceptible, dotted = resistant), with the SE indicated by the shaded regions based on individual curve fits for each coral genet. The ED50s of photochemical yield for each coral/phenotype were 36.1 °C M. capitata (susceptible), 36.6 °C M. capitata (resistant), 37.2 °C P. compressa (susceptible), 37.3 °C P. compressa (resistant). The ED50s of the color scores for each coral/phenotype were 34.2 °C M. capitata (susceptible), 34.5 °C M. capitata (resistant), 34.6 °C P. compressa (susceptible), 34.4 °C P. compressa (resistant).
Discussion
Legacy Effects of Successive Heatwaves Have Led to Annual Bleaching in Susceptible M. capitata.
Bleaching-susceptible colonies of M. capitata are now experiencing annual seasonal bleaching in the absence of anomalously high temperatures after a decade that included three marine heatwaves. This phenomenon was initially observed in the first summer following the 2015 heatwave and was likely exacerbated by the combined impacts of the back-to-back heatwaves in 2014 and 2015 (36, 37). Encouragingly, in the second year after that heatwave, bleaching-susceptible M. capitata regained pigmentation over the winter and did not bleach again the following fall, indicating a ~2-y recovery period. Yet, when faced with a third marine heatwave just 4 y later (2019), these same M. capitata colonies bleached again even though the heatwave was less severe. Declining performance in response to a second heatwave has been observed in corals from Australia (29) to the Caribbean (28); however, that bleaching-susceptible M. capitata colonies repeatedly bleached during each of the three summers following the 2019 event, in the absence of thermal stress, is alarming. While seasonal declines in coral biomass and symbiont density during summer temperatures are common (38, 39), they do not typically lead to the visually apparent bleaching observed here. These results indicate that the frequency of heatwaves in Hawai’i over the past decade has compounded the stress experienced by susceptible corals, leading to persistent declines in performance under ambient conditions. Furthermore, bleaching-susceptible individuals had higher partial mortality (~20%) than bleaching-resistant conspecifics (<5%) following the 2015 heatwave (33), underscoring the ecological significance of these differential responses. Encouragingly, bleaching-resistant individuals within this same population of M. capitata have remained consistently pigmented across multiple heatwaves and were 0.5 °C more heat tolerant than bleaching-susceptible conspecifics 3 y after the 2019 event. However, higher bleaching thresholds surprisingly did not translate into greater survival in the 3 y following the 2019 heatwave, indicating that the accumulation of stress following three successive heatwaves in under a decade negated the survival benefits of bleaching-resistance.
Physiological data further confirmed that resistance to bleaching in M. capitata was not a sufficient measure of coral performance. Specifically, we observed incomplete recovery across multiple phenotypic and physiological metrics in both bleaching-resistant and bleaching-susceptible individuals in the 3 y following the 2019 marine heatwave, highlighting a legacy of stress that transcended visual bleaching assessments and persisted for several years. For example, neither phenotype exhibited complete recovery in symbiont or host protein densities during the 3-y period following the 2019 heatwave. At their peak (after 35 mo of recovery), symbiont densities only reached up to half of preheatwave densities [January 2014: 4 to 5 × 106 cells cm−2 (40)]. Given the ongoing upward trajectory, symbiont recovery will likely continue so long as another heatwave does not occur. These results underscore that physiological recovery can be a multiyear process, even when visual recovery is apparent within a few weeks to months following heat stress (37, 41). Interestingly, not all physiological parameters demonstrated a lag in recovery, with tissue biomass and lipid densities displaying apparent recovery followed by strong seasonality in the first year post heat stress. However, traits important for coral fitness, such as growth and fecundity, can take 4 y or more to recover following heat stress (18, 42–44). Long-term investigations are clearly needed to observe recovery in metrics closely linked to fitness as marine heatwaves continue to increase in frequency and severity, and thus increasingly overlap with the time required by many corals to fully recover. Decreasing duration of periods of relief from thermal stress can compound physiological stress in many surviving corals, which may alter their relative performance and survival within communities (30) and will have important ramifications for community composition and ecosystem function.
A better understanding of the mechanisms driving bleaching resistance may help predict the fate of corals in future reefs. For example, thermal tolerance in M. capitata is associated with a combination of host (31) and symbiont (45) factors. In Kāne‘ohe Bay, M. capitata can host Cladocopium (ITS2-type “C31”) and Durusdinium glynnii (46–48), and bleaching resistance is strongly correlated with higher proportions of D. glynnii (45). Indeed, the bleaching-susceptible colonies observed here hosted almost exclusively Cladocopium spp., while bleaching-resistant colonies predominantly hosted D. glynnii (31, 49) (SI Appendix, Table S11). Both phenotypes exhibited additional physiological signatures of Cladocopium- or Durusdinium-dominated symbioses, respectively. For example, bleaching-susceptible M. capitata had nearly half as many symbionts as bleaching-resistant colonies across all seasons, matching observations that Cladocopium-dominated M. capitata tend to have lower symbiont densities than Durusdinium-dominated colonies (34, 37). While Durusdinium-dominated M. capitata were able to maintain bleaching resistance and higher symbiont densities, D. glynnii generally provides the host with fewer resources than Cladocopium spp. under both ambient and heat stress conditions (50), indicating that symbiont retention during heat stress is an incomplete measure of coral performance. However, the nutritional benefits of hosting Cladocopium spp. may diminish over time as heatwaves and bleaching become increasingly common, and the impacts of changing symbiont dominance on coral growth and survival, and thus ecosystem function, require further study.
Environmental Memory of Heatwaves Has Led to Beneficial Acclimatization in P. compressa.
Bleaching-susceptible and bleaching-resistant P. compressa appear to have converged on the same resistant phenotype despite past differences in bleaching susceptibility. Surprisingly, neither phenotype bleached significantly during the 2019 heatwave, even though the bleaching-susceptible colonies exhibited severe bleaching and some (~20%) partial mortality during the 2015 heatwave (33, 34). While lower bleaching severity in 2019 may have been due to somewhat lower levels of heat stress accumulation than in 2015, experimental heat stress tests confirmed identical bleaching thresholds in bleaching-susceptible and bleaching-resistant phenotypes, and neither phenotype experienced significant mortality following the 2019 event. Together, these results indicate that beneficial acclimatization occurred and has persisted for several years in this species. Higher bleaching thresholds have been observed in multiple coral species following successive marine heatwaves (17–21), supporting the hypothesis that environmental memory of a prior stress event improves the response to a subsequent exposure, and is a common capability in corals. Increases in bleaching resistance from 2015 to 2019 were also observed in P. compressa across the population, which may also have stemmed from beneficial acclimatization; however, selection against weak corals or symbioses (20, 23, 30) or a shift to more stress-tolerant symbionts (30) is also possible. Support for these latter two hypotheses is limited for P. compressa in Kāne‘ohe Bay, where i) whole-colony mortality was low in the aftermath of the 2014 and 2015 heatwaves (33, 36), ii) P. compressa does not exhibit evidence of cryptic host speciation (51), and iii) P. compressa maintains a specific symbiosis with a single symbiont species, Cladocopium ITS2-type “C15” (46). By following individual colonies across multiple heatwaves, this study indicates that the heatwaves in 2014 and 2015 resulted in stress hardening, not loss of sensitive individuals from the population, that first manifested as a decrease in bleaching severity in 2019, and this benefit has persisted within individuals for nearly a decade. While the exact mechanisms conferring this resilience have not been determined, physiological plasticity (18, 37), constitutive upregulation of stress-response genes (52, 53), and epigenetic modifications (54) all likely contribute and represent important avenues of future study. Importantly, whether the benefits of environmental memory of moderate heat stress will persist as heatwaves become more intense remains unknown.
In the years following the 2019 heatwave, both phenotypes of P. compressa exhibited physiological recovery across multiple traits, requiring ~1.5 y to reach symbiont, chlorophyll-a and protein densities similar to historical preheatwave levels (55). Given their faster recovery and elevated bleaching thresholds (+0.7 to 1.2 °C) relative to M. capitata, P. compressa may become more dominant in this region as heatwaves become more frequent. Indeed, M. capitata exhibited greater partial mortality (Fig. 2) and greater declines in benthic cover than P. compressa following the 2019 event (56), although both species experienced significant mortality across Kāne‘ohe Bay in 2019 [19% decline in P. compressa, 23% decline in M. capitata (56)]. Greater bleaching resistance and lower mortality favors the dominance of P. compressa, which already reaches >75% cover on some reefs in Kāne‘ohe Bay (57). While high coral cover persists in this location despite bleaching-related mortality of these two dominant species, and has historically been considered a key metric of reef condition, the loss of biodiversity associated with transitioning from a multispecies assemblage to a predominantly P. compressa landscape would likely have a multitude of adverse effects on ecosystem function, from declining coral productivity to losses of blue food security (10, 58). Further, critical processes such as reef accretion are projected to become uncoupled from coral cover under global change (59). Here, P. compressa was unable to sustain skeletal density as high as M. capitata, despite maintaining up to three times higher coral cover and exhibiting less bleaching and mortality. These patterns may be explained by: i) enhanced heat tolerance resulting in trade-offs with growth [(60, 61 but see ref. 62], and/or ii) the duration of our study was not long enough to capture the recovery of secondary calcification (i.e., densification) for this species, as recurring marine heatwaves could have delayed the revival of densification. Indeed, P. compressa are unable to recover calcification rates in the first 8 mo following heat stress (63), and elsewhere in the Pacific, Porites spp. can exhibit growth hiatuses for up to 4 y in the aftermath of a marine heatwave (44). Whether modern reefs are able to maintain net accretion and continue to provide the critical ecosystem services humans rely on remains to be seen.
Conclusions.
We have entered a new era of ocean warming that is affecting coral reefs in ways we are only just beginning to understand. Corals in the Main Hawaiian Islands have experienced a rise in offshore sea-surface temperatures of 1.15 °C in the last 60 y (64, 65), leading to an unprecedented three coral bleaching events in the last decade (2014 to 2023) that were preceded by a single widespread coral bleaching event (1996) the entire century prior (57, 66). The responses of individual corals to these increasingly frequent heatwaves demonstrate the divergent intra- and interspecific bleaching and recovery trajectories possible within a single coral community, highlighting the challenge of predicting future coral performance in a changing ocean. In one direction, P. compressa that were highly sensitive to marine heatwaves in 2014 and 2015 (i.e., severely bleached) have become visually and physiologically indistinguishable from bleaching-resistant conspecifics during a third heatwave (2019) and during extreme heat stress tests, indicating beneficial acclimatization that persists across many years. This increase in bleaching resistance was accompanied by rapid physiological recovery after repeat heat stress, although there remained evidence of persistent stress (e.g., weakened skeletons), suggesting that these corals may undergo tradeoffs that can erode ecosystem function. Increases in coral bleaching resistance across recurring heatwaves have become more prevalent on reefs across the globe (15, 17, 19, 21), which is encouraging, as avoiding bleaching is often associated with greater survival (33) and stress hardening may thus promote the persistence of corals in our warming oceans. On the other hand, the extent of coral mortality has been increasing with each successive heatwave in this system, from <1% in 1996 to 13% in 2014, 22% in 2015, and >20% in 2019 (56, 67). Furthermore, bleaching-susceptible M. capitata are visibly struggling from the recent barrage of heatwaves, manifesting as annual seasonal bleaching and escalating partial mortality in the absence of measurable heat stress. Importantly, even bleaching resistance was not associated with greater survival or recovery capacity in M. capitata, highlighting the danger of predicting future individual performance and reef function from a lack of visual bleaching alone. The inability of M. capitata of either bleaching phenotype to recover physiologically after 3 y following a repeat heatwave underscores that coral resilience is a multifaceted trait beyond bleaching resistance (60) and brings into question how we define coral resilience in the context of global change. Now, more than ever, seasonal and long-term studies are critically needed to identify corals that cannot just withstand and survive repeated heat stress events, but also rapidly recover ecosystem-defining traits (e.g., biomineralization) to continue providing the critical ecosystem services coastal communities directly rely on. Urgent, collective global action to eliminate greenhouse gas emissions remains the only approach that may provide sufficient time for corals to acclimatize and adapt to rapid climate-induced temperature increases in order for coral reef ecosystems to persist in the Anthropocene.
Materials and Methods
Study Site and Seawater Temperature.
This study was conducted at PR13 in the southern region of Kāne’ohe Bay, O’ahu, Hawai’i. Seawater temperatures were recorded from January 2014 to April 2023. Where the record was incomplete, temperature data from two nearby reefs (<0.5 km away) were included. Cumulative heat stress (DHW) was determined following the equations in ref. 68 using the maximum monthly mean (MMM) of 27.3 °C. These results were compared to DHW on the reef of Moku o Lo’e (PR1), and vs. DHW using the regional MMM of 27.0 °C and MMM used in the literature for Kāne’ohe Bay [27.7 °C (37); 28.0°C (34, 69)] (SI Appendix).
Coral Bleaching and Mortality Assessments.
Adjacent conspecific corals with contrasting bleaching phenotypes (i.e., bleaching-resistant vs. bleaching-susceptible) were first identified during the 2015 marine heatwave (33). A total of 10 colonies per phenotype per species of M. capitata and P. compressa were followed for this study. Colonies of M. capitata were previously confirmed to be distinct genotypes (31), and P. compressa has very low clonality in this area (70). Bleaching severity and partial mortality of each colony was determined from photographs taken between October 2015 to April 2023 (SI Appendix). Coral cover and reef-wide bleaching prevalence were also determined at each time point from benthic photoquadrats as in refs. 33 and 34 (SI Appendix).
Physiological Analyses.
Fragments from each colony were collected across eight time points from October 2019 to September 2022. Photosynthetic rates were assessed at increasing irradiance via changes in oxygen evolution as previously described (34) (SI Appendix, Fig. S8). Coral fragments were then flash-frozen in liquid nitrogen and stored at −80 °C until further processing. Coral tissue was removed from skeletons with a waterpik, and host and symbiont fractions were separated by differential centrifugation. All details of host (tissue biomass, protein and lipid concentrations, TAC, melanin content, PPO activity, and CaCO3 density) and symbiont (symbiont density and chlorophyll-a concentration) analyses can be found in SI Appendix.
Acute Heat Stress Experiment.
Ten individual colonies of each phenotype per species were sampled at the end of summer (September 1, 2022) and assessed for heat tolerance following a standardized protocol (35, 71). Briefly, corals were exposed to a 3-h ramp to respective treatment temperatures (ambient: 28.0 °C; amb+3 °C: 31.0 °C, amb+6 °C: 34.0 °C, and amb+9 °C: 37.0 °C), a 3-h hold, and a 1-h ramp down to ambient. At the end of the ramp and 1 h after sunset (~19:30), corals were assessed for photochemical yield (Fv/Fm). The following morning corals were photographed with a color standard to assess coral color (Fig. 6 and SI Appendix).
Statistical Analyses.
All statistical analyses were done using R version 4.0.3 software (72) and are explained in detail in SI Appendix, Table S12. Briefly, a linear model was used to test for differences in colony-level bleaching severity between species, phenotype, season, and time. Linear mixed effects models were used to assess for differences in physiological parameters. Differences in coral multivariate phenotypes were analyzed separately for each coral species using PERMANOVA and principal component analysis, with the fixed effects phenotype and months post heat stress. To determine how heat tolerance differed among coral species and phenotype, dose–response curves were fit to the median Fv/Fm across temperature treatments, and the effective temperature to induce a 50% loss in Fv/Fm (ED50) was calculated as the Fv/Fm or color score from the model fit that is 50% of the initial value (71).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank the staff of the Hawai’i Institute of Marine Biology for logistical support, especially marine safety officer Jason Jones. We also thank Chris Suchocki, Ariana Huffmyer, Wesley Sparagon, Mariana Rocha de Souza, and Joshua Hancock for field assistance. Corals were collected under the following permits from the State of Hawai’i’s Division of Aquatic Resources: SAP-2022-28, SAP-2021-41, and SAP-2020-41. This work was supported by NSF awards OCE-1923743 and OCE-2102989 to K.L.B., OCE-1949033 to C.E.N., and OCE-1756623 and OCE-2103067 to H.M.P. This paper was also funded in part by a grant/cooperative agreement from the National Oceanic and Atmospheric Administration (NOAA), Project A/AS-1 which is sponsored by the University of Hawaii Sea Grant College Program, SOEST, under Institutional Grant No. NA22OAR4170108 from NOAA Office of Sea Grant, Department of Commerce. The views expressed herein are those of the author(s) and do not necessarily reflect the views of NOAA or any of its subagencies.
Author contributions
K.T.B., H.M.P., and K.L.B. designed research; K.T.B., E.A.L., B.H.G., E.K., R.M., C.D., and K.L.B. performed research; K.T.B., C.E.N., and K.L.B. analyzed data; and K.T.B. and K.L.B. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All data have been deposited and are available at National Science Foundation (NSF) Biological and Chemical Oceanography Data Management Office (BCO-DMO) (https://www.bco-dmo.org/project/868513) (73). All R scripts and analyses are available on GitHub (https://github.com/imkristenbrown/Divergent-bleaching-and-recovery-trajectories-in-corals-following-of-heatwaves). (74).
Supporting Information
References
- 1.Hughes T. P., et al. , Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Hoegh-Guldberg O., et al. , The human imperative of stabilizing global climate change at 1.5 °C. Science 365, eaaw6974 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Frölicher T. L., Fischer E. M., Gruber N., Marine heatwaves under global warming. Nature 560, 360–364 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Oliver E. C. J., et al. , Marine heatwaves. Ann. Rev. Mar. Sci. 13, 313–342 (2021). [DOI] [PubMed] [Google Scholar]
- 5.van Woesik R., et al. , Coral-bleaching responses to climate change across biological scales. Glob. Chang. Biol. 28, 4229–4250 (2022), 10.1111/gcb.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muscatine L., Porter J. W., Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460 (1977). [Google Scholar]
- 7.Muscatine L., L. R. McCloskey, R. E. Marian, Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 26, 601–611 (1981). [Google Scholar]
- 8.Knowlton N., et al. , “Coral reef biodiversity” in Life in the World’s Oceans: Diversity, Distribution, and Abundance, A. D. McIntyre, Ed. (John Wiley and Sons, 2010), pp. 65–77. [Google Scholar]
- 9.Wild C., et al. , Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshwater Res. 62, 205–215 (2011). [Google Scholar]
- 10.Worm B., et al. , Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Oliver E. C. J., et al. , Projected marine heatwaves in the 21st century and the potential for ecological impact. Front. Marine Sci. 6, 734 (2019). [Google Scholar]
- 12.Smale D. A., et al. , Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 9, 306–312 (2019). [Google Scholar]
- 13.Hackerott S., Martell H. A., Eirin-Lopez J. M., Coral environmental memory: Causes, mechanisms, and consequences for future reefs. Trends Ecol. Evol. 36, 1011–1023 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Brown K. T., Barott K. L., The costs and benefits of environmental memory for reef-building corals coping with recurring marine heatwaves. Integr. Comp. Biol. 62, 1748–1755 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Brown B. E., Dunne R. P., Edwards A. J., Sweet M. J., Phongsuwan N., Decadal environmental “memory” in a reef coral? Mar. Biol. 162, 479–483 (2015). [Google Scholar]
- 16.Brown B. E., Dunne R. P., Goodson M. S., Douglas A. E., Bleaching patterns in reef corals. Nature 404, 142–143 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Gintert B. E., et al. , Marked annual coral bleaching resilience of an inshore patch reef in the Florida Keys: A nugget of hope, aberrance, or last man standing? Coral Reefs 37, 533–547 (2018). [Google Scholar]
- 18.Fisch J., Drury C., Towle E. K., Winter R. N., Miller M. W., Physiological and reproductive repercussions of consecutive summer bleaching events of the threatened Caribbean coral Orbicella faveolata. Coral Reefs 38, 863–876 (2019). [Google Scholar]
- 19.Hughes T. P., et al. , Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Chang. 9, 40–43 (2019). [Google Scholar]
- 20.Pratchett M. S., McCowan D., Maynard J. A., Heron S. F., Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS One 8, e70443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guest J. R., et al. , Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One 7, e33353 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S. C., Johnston E. C., Wyatt A. S. J., Leichter J. J., Edmunds P. J., Response diversity in corals: Hidden differences in bleaching mortality among cryptic Pocillopora species. Ecology 102, e03324 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley K. M., et al. , Symbioses are restructured by repeated mass coral bleaching. Sci. Adv. 8, eabq8349 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson D. M., van Woesik R., Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc. Biol. Sci. 276, 2893–2901 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sully S., Burkepile D. E., Donovan M. K., Hodgson G., van Woesik R., A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, 1264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley K. M., Bay L. K., van Oppen M. J. H., The active spread of adaptive variation for reef resilience. Ecol. Evol. 9, 11122–11135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmunds P. J., Gates R. D., Acclimatization in tropical reef corals. Mar. Ecol. Prog. Ser. 361, 307–310 (2008). [Google Scholar]
- 28.Neal B. P., et al. , Caribbean massive corals not recovering from repeated thermal stress events during 2005–2013. Ecol. Evol. 7, 1339–1353 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalton S. J., et al. , Successive marine heatwaves cause disproportionate coral bleaching during a fast phase transition from El Niño to La Niña. Sci. Total Environ. 715, 136951 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Grottoli A. G., et al. , The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Chang. Biol. 20, 3823–3833 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Drury C., et al. , Intrapopulation adaptive variance supports thermal tolerance in a reef-building coral. Commun. Biol. 5, 486 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putnam H. M., Avenues of reef-building coral acclimatization in response to rapid environmental change. J. Exp. Biol. 224, jeb239319 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Matsuda S., et al. , Coral bleaching susceptibility is predictive of subsequent mortality within but not between coral species. Front. Ecol. Evol. 8, 1–14 (2020). [Google Scholar]
- 34.Innis T., et al. , Marine heatwaves depress metabolic activity and impair cellular acid-base homeostasis in reef-building corals regardless of bleaching susceptibility. Glob. Chang. Biol. 27, 2728–2743 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Voolstra C. R., et al. , Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Chang. Biol. 26, 4328–4343 (2020), 10.1111/gcb.15148. [DOI] [PubMed] [Google Scholar]
- 36.Ritson-Williams R., Gates R. D., Coral community resilience to successive years of bleaching in Kane ‘ohe Bay, Hawai’i. Coral Reefs 39, 757–769 (2020). [Google Scholar]
- 37.Wall C. B., et al. , Shifting baselines: Physiological legacies contribute to the response of reef corals to frequent heatwaves. Funct. Ecol. 35, 1366–1378 (2021). [Google Scholar]
- 38.Fitt W. K., McFarland F. K., Warner M. E., Chilcoat G. C., Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685 (2000). [Google Scholar]
- 39.Thornhill D. J., et al. , A connection between colony biomass and death in Caribbean reef-building corals. PLoS One 6, e29535 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbin E. M., Putnam H. M., Gates R. D., Nitschke M. R., Davy S. K., Species-specific differences in thermal tolerance may define susceptibility to intracellular acidosis in reef corals. Mar. Biol. 162, 717–723 (2015). [Google Scholar]
- 41.Thomas L., Palumbi S. R., The genomics of recovery from coral bleaching. Proc. Biol. Sci. 284, 20171790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward S., Harrison P., Hoegh-Guldberg O., “Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress” in 9th International Coral Reef Symposium, Moosa M. K., et al., (Ministry of Environment: Indonesian Institute of Sciences: International Society for Reef Studies, Bali, 2002), Citeseer. [Google Scholar]
- 43.Levitan D. R., Boudreau W., Jara J., Knowlton N., Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar. Ecol. Prog. Ser. 515, 1–10 (2014). [Google Scholar]
- 44.Cantin N. E., Lough J. M., Surviving coral bleaching events: Porites growth anomalies on the Great Barrier Reef. PLoS One 9, e88720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunning R., Ritson-Williams R., Gates R. D., Patterns of bleaching and recovery of Montipora capitata in Kāne ‘ohe Bay, Hawai’i, USA. Mar. Ecol. Prog. Ser. 551, 131–139 (2016). [Google Scholar]
- 46.Wham D. C., Ning G., LaJeunesse T. C., Symbiodinium glynnii sp. nov., a species of stress-tolerant symbiotic dinoflagellates from pocilloporid and montiporid corals in the Pacific Ocean. Phycologia 56, 396–409 (2017). [Google Scholar]
- 47.LaJeunesse T. C., et al. , High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs 23, 596–603 (2004). [Google Scholar]
- 48.de Souza M. R., et al. , Importance of depth and temperature variability as drivers of coral symbiont composition despite a mass bleaching event. Sci. Rep. 13, 8957 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dilworth J., Caruso C., Kahkejian V. A., Baker A. C., Drury C., Host genotype and stable differences in algal symbiont communities explain patterns of thermal stress response of Montipora capitata following thermal pre-exposure and across multiple bleaching events. Coral Reefs 40, 151–163 (2021). [Google Scholar]
- 50.Allen-Waller L., Barott K. L., Symbiotic dinoflagellates divert energy away from mutualism during coral bleaching recovery. Symbiosis 89, 173–186 (2023). [Google Scholar]
- 51.Forsman Z. H., Ritson-Williams R., Tisthammer K. H., Knapp I. S. S., Toonen R. J., Host-symbiont coevolution, cryptic structure, and bleaching susceptibility, in a coral species complex (Scleractinia; Poritidae). Sci. Rep. 10, 16995 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barshis D. J., et al. , Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. U.S.A. 110, 1387–1392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellantuono A. J., Granados-Cifuentes C., Miller D. J., Hoegh-Guldberg O., Rodriguez-Lanetty M., Coral thermal tolerance: Tuning gene expression to resist thermal stress. PLoS One 7, e50685 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dixon G., Liao Y., Bay L. K., Matz M. V., Role of gene body methylation in acclimatization and adaptation in a basal metazoan. Proc. Natl. Acad. Sci. U.S.A. 115, 13342–13346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues L. J., Grottoli A. G., Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 52, 1874–1882 (2007). [Google Scholar]
- 56.Yadav S., et al. , Fine-scale variability in coral bleaching and mortality during a marine heatwave. Front. Marine Sci. 10 (2023). [Google Scholar]
- 57.Bahr K. D., Jokiel P. L., Toonen R. J., The unnatural history of Kāne’ohe Bay: Coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3, e950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clements C. S., Hay M. E., Biodiversity has a positive but saturating effect on imperiled coral reefs. Sci. Adv. 7, eabi8592 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dove S. G., Brown K. T., Van Den Heuvel A., Chai A., Hoegh-Guldberg O., Ocean warming and acidification uncouple calcification from calcifier biomass which accelerates coral reef decline. Commun. Earth Environ. 1, 1–9 (2020). [Google Scholar]
- 60.Walker N. S., Nestor V., Golbuu Y., Palumbi S. R., Coral bleaching resistance variation is linked to differential mortality and skeletal growth during recovery. Evol. Appl. 16, 504–517 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornwell B., et al. , Widespread variation in heat tolerance and symbiont load are associated with growth tradeoffs in the coral Acropora hyacinthus in Palau. Elife 10, e64790 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lachs L., et al. , No apparent trade-offs associated with heat tolerance in a reef-building coral. Commun. Biol. 6, 400 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodrigues L. J., Grottoli A. G., Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta 70, 2781–2789 (2006). [Google Scholar]
- 64.Coles S. L., et al. , Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ 6, e5347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bahr K. D., Jokiel P. L., Rodgers K. S., The 2014 coral bleaching and freshwater flood events in Kāne’ohe Bay, Hawai’i. PeerJ 3, e1136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jokiel P. L., Brown E. K., Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob. Chang. Biol. 10, 1627–1641 (2004). [Google Scholar]
- 67.Bahr K. D., Rodgers K. S., Jokiel P. L., Impact of three bleaching events on the reef resiliency of Kāne‘ohe Bay, Hawai’i. Front. Marine Sci. 4, 398 (2017). [Google Scholar]
- 68.Brown K. T., Eyal G., Dove S. G., Barott K. L., Fine-scale heterogeneity reveals disproportionate thermal stress and coral mortality in thermally variable reef habitats during a marine heatwave. Coral Reefs 42, 131–142 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jury C. P., Toonen R. J., Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proc. R Soc. B Biol. Sci. 286, 20190614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Locatelli N. S., Drew J. A., Population structure and clonal prevalence of scleractinian corals (Montipora capitata and Porites compressa) in Kaneohe Bay, Oahu. bioRxiv [Preprint] (2019). 10.1101/2019.12.11.860585. Accessed 13 August 2023. [DOI]
- 71.Evensen N. R., et al. , Empirically derived thermal thresholds of four coral species along the Red Sea using a portable and standardized experimental approach. Coral Reefs 41, 239–252 (2022). [Google Scholar]
- 72.R Core Team, R: A language and environment for statistical computing (Version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria, 2021). https://www.R-project.org/. Accessed 10 October 2020.
- 73.Brown K. T., et al. , Project: RAPID: Collaborative Research: Disentangling the effects of heat stress versus bleaching phenotype on coral performance. Biological and Chemical Oceanography Data Management Office (BCO-DMO). https://www.bco-dmo.org/project/868513. Deposited 28 November 2023.
- 74.K. T. Brown et al. , Divergent-bleaching-and-recovery-trajectories-in-corals-following-of-heatwaves. GitHub. https://github.com/imkristenbrown/Divergent-bleaching-and-recovery-trajectories-in-corals-following-of-heatwaves. Deposited 10 November 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All data have been deposited and are available at National Science Foundation (NSF) Biological and Chemical Oceanography Data Management Office (BCO-DMO) (https://www.bco-dmo.org/project/868513) (73). All R scripts and analyses are available on GitHub (https://github.com/imkristenbrown/Divergent-bleaching-and-recovery-trajectories-in-corals-following-of-heatwaves). (74).