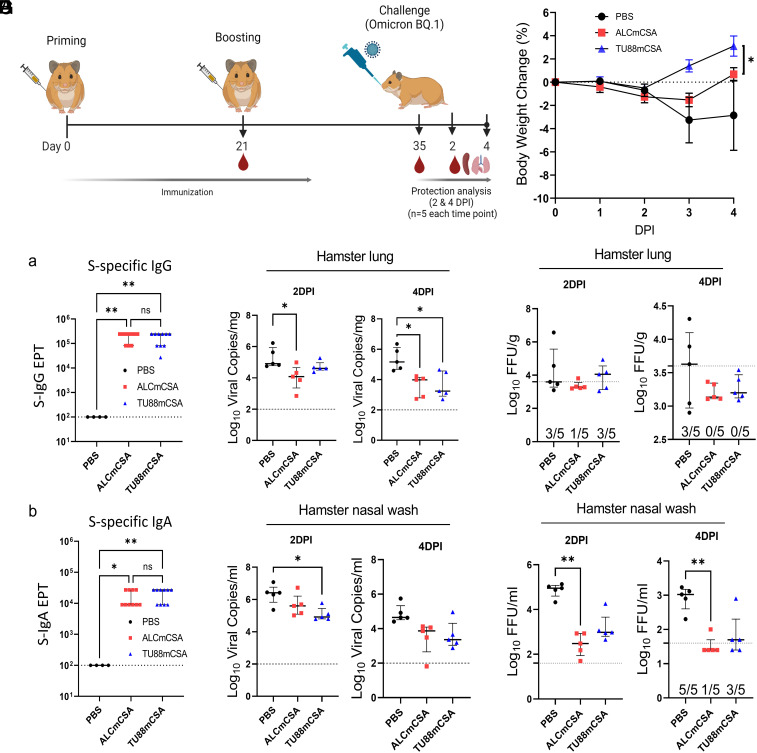
Fig. 5.
ALCmCSA and TU88mCSA vaccination confer protection against challenge with the SARS-CoV-2 Omicron BQ.1 in hamsters. (A) Schematic illustration of hamster challenge study. Three groups of hamsters (n = 10 per group) were vaccinated I.M. with mock (PBS), ALCmCSA (5 µg for each) or TU88mCSA (5 µg for each) at weeks 0 and 3, followed by I.N. challenge with SARS-CoV-2 Omicron BQ.1 strain (2 × 104 pfu) at week 5. On 2 (n = 5) and 4 DPI (n = 5), lung tissues were harvested for analysis of viral titers; nasal washes were collected for analysis of viral titer; hamster body weights were also monitored. (B) A comparison of hamster body weight changes is shown between different groups from 0 to 4 DPI. Significance was statistically determined by the two-way ANOVA Tukey test, *P < 0.05. Average ± SD (n = 5 mice per group). (C) Analysis of S-specific IgG (a) and IgA (b) EPT in the hamster serum at week 5 post ALCmCSA or TU88mCSA immunization (mock, n = 4; vaccine group, n = 10). (D) Comparison of viral RNA copies in hamster lungs (log10 viral copies per milligram) between mock and vaccine groups are shown for tissues collected on 2 and 4 DPI. (E) Comparisons of viral titers in the hamster lungs (log10 FFU per gram) between mock and vaccine group are shown for tissues collected on 2 and 4 DPI. (F) A comparison of viral RNA copies in the nasal washes (log10 viral copies per milliliter) is shown between the indicated groups on 2 and 4 DPI. (G) Comparisons of viral titers in the hamster nasal washes (log10 FFU per milliliter) between the mock and vaccine groups are shown for samples collected on 2 and 4 DPI. Unless specified otherwise, significance for (C) and (D–G) was statistically determined by the one-way ordinary ANOVA Tukey test, ns, no significance, *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001. Average ± SD. biological replicates shown.