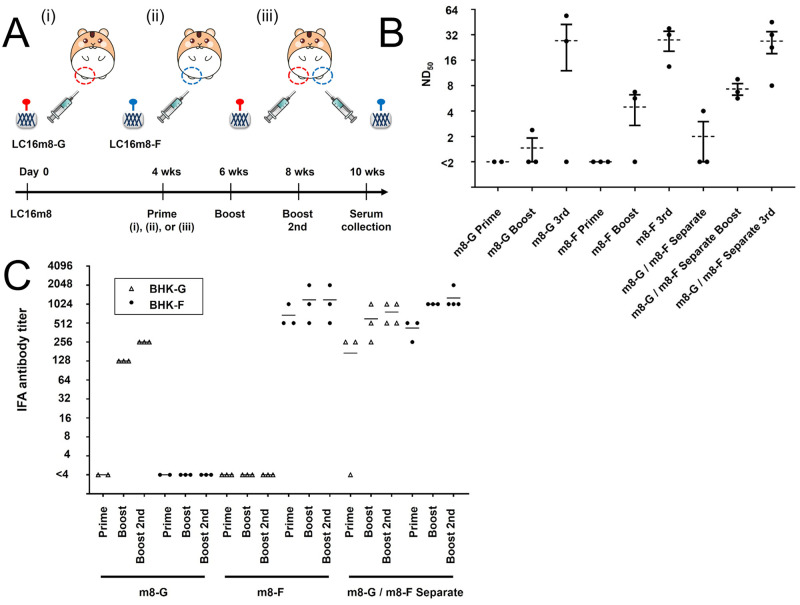
Fig 4. Effective induction of neutralizing antibodies by three immunization cycles with the recombinant LC16m8s in the VV-preimmunized hamsters.
(A) Experimental design and schedule of inoculations with LC16m8 and the recombinant LC16m8s. Twenty-seven hamsters were intramuscularly inoculated with 1×106 PFU of the LC16m8. At 4 weeks, three groups of hamsters (n = 8, 9 or 10) were inoculated with 2×106 PFU of the LC16m8-G (Group 1: VV-G-single-site), 2×106 PFU of the LC16m8-F (Group 2: VV-F-single-site) or both viruses (1×106 PFU each, onto two sites) (Group 3: VV-G-F-separate-sites). Two weeks after infection with the recombinant LC16m8s, one-third of the hamsters in each group were inoculated once or twice at a 2-week interval with the same recombinant viruses as the prime inoculation. Two weeks after the last shot, a serum sample was collected from each hamster. All groups of hamsters are shown with roman numerals in parentheses. Neutralizing titers were determined using live NiV (B). Each circled dot represents the titer (ND50) of each serum sample. Dotted line and bars show mean ± SEM. A one-tailed Student’s t test assuming unequal variance was used for analyzing the data. ND50 values below the LOD (<2) were assigned a value of 1. Antibody titers were also determined using the IFA method (C). BHK cells were transfected with the expression plasmid encoding NiV-G or NiV-F. At 24–36 hpi, the cells were fixed and reacted with dilutions of the hamster serum, followed by incubation with Alexa Fluor 488-conjugated anti-hamster secondary antibody. The stained cells were observed under a fluorescence microscope. IFA titers were defined based on the maximum serum dilution in which a fluorescence signal was detected, and the titers measured in BHK cells expressing NiV-G (BHK-G) or BHK cells expressing NiV-F (BHK-F) are indicated by a triangle or circle dot, respectively.