Abstract
Rotator cuff injury is the leading cause of shoulder pain. Hyperlipidemia is responsible in depositing lipids in tendons and reduce the healing upon injuries or tears. In this study, we created rotator cuff injury and repair models in swine and studied the changes in biomechanical properties of infraspinatus tendons in hyperlipidemic swine. The infraspinatus tendons from control group, hyperlipidemic injury and repair group of animals were collected and tested ex-vivo. The ultimate tensile strength (UTS) and modulus of elasticity increased in the tendons from the contralateral side on both the injury and repair models and were higher than the injury side. The presence of large number of fibrous tissues in the surgical site of repair and increased water content was observed in addition to the fatty infiltration which would have contributed to the decreased mechanical properties of the injured tendons following repair. Meanwhile the tendons of the contralateral side in both the injury and repair model showed adaptation to chronic load as observed in the modulus and viscoelastic properties. This is a pilot study that warrants detailed investigation in a larger sample size with longer duration following tendon injury and repair to gain better understanding on the effect of hyperlipidemia in the healing of rotator cuff tendon injury.
Keywords: Biomechanical properties, Dynamic modulus, Hyperlipidemia, Infraspinatus tendon, Rotator cuff injury model
1. Introduction
Rotator cuff tear is the most common shoulder disorder and may be symptomatic or asymptomatic. The prevalence and failure of surgical repair of rotator cuff is on the rise with a failure rate of surgical repair up to 20–94% [1–3]. Age, physical activities, diabetes, smoking, body weight and chronic injuries are the factors that affect the effective healing of rotator cuff injuries. Hyperlipidemia has its enduring association with rotator cuff injury and its impact in reduced healing, but the mechanism is largely unknown [4,5]. Hyperlipidemia decreases the effective outcome of the treatment procedures and renders inadequate healing [5,6]. Injury or tear to the rotator cuff tendon disrupts the function of tendon in transmitting the load from muscles to bones leading to pain and impaired mechanical function [3]. Hyperlipidemia causes the infiltration of lipids into the tendons and with increasing age led to reduced mechanical properties of the tendons [7].
To understand the changes in mechanical properties associated with pathological conditions, ex-vivo testing of tissues are important to develop biomaterials and treatment strategies [8]. Since clinical outcomes are not consistent in bringing out the association between hyperlipidemia and rotator cuff tendon injuries and its healing, translational studies are required to understand the prognosis of tear/injury and the treatment outcome [9,10].
In this study we report the changes in mechanical properties of infraspinatus tendon of the rotator cuff of hyperlipidemic swine that underwent rotator cuff injury with and without repair surgery. Our goal was to study the differences in biomechanical properties of the infraspinatus tendon ex-vivo for the injury and repair model of hyperlipidemic swine. Hence, we have not included exercise and physical activities in our study to emulate the normal rotator cuff injury and repair under the influence of hyperlipidemia.
2. Materials and Methods
2.1. Tendon tissue collection and preparation
The Institutional Animal Care and Use Committee (IACUC) of Western University of Health Sciences, Pomona, CA, USA approved the experimental research protocol (R22IACUC034).
Seven female Yucatan miniswine (Sus scrofa), 26–30 kg, 30-wks old, were purchased from Premier bioresources, CA, USA. Swine were acclimatized to 12/12 hours of light-dark cycle. Three swine were fed twice every day regular pig diet (Group I - control) and four swine were fed with high-cholesterol-high-fat diet to develop hyperlipidemia [11,12]. Rotator cuff injury (Group II - injury) was made on two hyperlipidemic swine and the other two hyperlipidemic swine had tendon injury and repair by Mason-Allen suture technique (Group III – injury + repair).
Briefly, under general anesthesia the incision was made on the left shoulder and the infraspinatus was exposed and braided nonabsorbable polyurethane suture was placed through the infraspinatus tendon and the tendon was surgically detached from the greater tuberosity. For the repair model (Group-III) the detached tendons were repaired with Mason-Allen suture technique, while in injury model (Group-II) the detached tendon with suture was left as such for identification. After surgery the incision was sutured to close the wound. After recovery from anesthesia the swine were moved to pen and medication for pain management was administered for 3 days or till the animals shows no signs of pain. The swine were allowed to move freely in the pen without restriction of movements and diet as described above.
After 8 weeks the animals were sacrificed, and the infraspinatus tendon tissues of the rotator cuff were collected from the surgical injury side (IS) and the contralateral side (CS) of the hyperlipidemic swine and the control swine. The tissues were wrapped in tissue paper pre-soaked with PBS and stored at −80°C until the day of testing. On the day of testing the tendon tissues were thawed using a two- step protocol: (i) 4 h at 4°C, and (ii) 2 h at room temperature [13]. After thawing, two equal pieces from the tendon core were manually cut using scalpel blade. The cut pieces were approximately 55 mm in length for tensile testing and Dynamic mechanical analysis (DMA) and kept at 4°C to maintain the freshness of tissue until testing. The tendon tissues at the repair site of the repair model had dense fibrous tissue and that made the extraction of the tendon more difficult than the injury side of the injury model.
2.2. Tensile testing
The tensile testing was conducted on 3 tendons from group I control animals, 4 tendons (2-CS and 2-IS) from group II, 4 tendons (2-CS and 2-IS) from group III were used and compared the tensile strengths in 8 tendons (4-CS and 4-IS) from both group II and group III animals. Approximately 55 mm of the tendon core were cut, and the length, width and thickness were measured using digital vernier caliper. The smaller dimensions were used in calculating the cross-sectional area. The tendons were secured in between sandpaper and mounted using the tension grips on to TA Electroforce 3300 (TA Instruments, New Castle, DE, USA) equipped 1000 N load cell. The gauge length was 30 mm. The samples were preconditioned using for 10 cycles at a frequency of 1.0 Hz between 0 and 10 N under load control [14]. Following preconditioning, the tendon tissue samples were ramped to failure at a crosshead speed set to 100µm/s while recording the force and displacement continuously. Failure was defined as the decrease in load below 20% of the maximum load. The engineering stress (ζ) and engineering strain (ε) were determined, and the stress-strain curve was plotted. The modulus of elasticity (E) was obtained from the slope of the linear region of the stress-strain curve. The ultimate tensile strength (UTS) and the strain at failure were also calculated [15,16].
2.3. Dynamic mechanical analysis
For dynamic mechanical analysis (DMA) 3 tendons from group I control animals, 4 tendons (2-CS and 2-IS) from group II, 4 tendons (2-CS and 2-IS) from group III were used and compared the DMA in 8 tendons (4-CS and 4-IS) from both group II and group III animals. The tendons were secured in between sandpaper and mounted using the tension grips on to TA Electroforce 3300 (TA Instruments, New Castle, DE, USA) equipped with 1000 N load cell. An initial load of 2.0 N was applied to remove the slack and the gauge length was recorded. The tendon tissues were preconditioned with 10 cycles at a frequency of 1.0 Hz between 0 and 10 N under load control. Sinusoidal loading between 10 N and 20 N was applied and two frequency sweep was performed at (i) 0.2 Hz to 2.0 Hz with an increase in frequency of 0.2Hz until 2 Hz, and (ii) 1 Hz to 41 Hz with increase in frequency of 10 Hz until 41 Hz. Throughout the testing, the samples were kept immersed in PBS at room temperature to prevent excess drying. The viscoelastic properties, dynamic modulus (E*), storage modulus (E’), loss modulus (E”), and damping ability (Tan δ) were calculated and recorded by the machine [13,14].
2.4. Water content
The water content of the tendon core (10 mm length, 3–4 mm width and thickness) was calculated based on the weight of tendon tissues before (Wwet) and after drying (Wdry) for 72 hours at 40oC [13]. The samples were weighed using analytical balance with the resolution of 0.01 mg. The water content was calculated using the below formula:
2.5. Histology
Tendon tissue samples were fixed with 10% buffered formalin for 72 hours, processed, embedded in paraffin, and 7 µm thick sections were obtained. The sections were stained with hematoxylin and eosin (H&E) and analyzed for cellularity, ECM organization, and tendon alignment.
2.6. Statistical Analysis
The statistical analyses of the data between the control group (Group I; N=3) and the contralateral side uninjured tendon of the hyperlipidemic swine (Group II and III, N=4), injured (Group II, N=2) and injured followed by repair in hyperlipidemic swine (Group III, N=2) were performed by unpaired Student t-test for UTS, modulus of elasticity, percent failure strain, and water content. The statistical analysis for dynamic mechanical analysis was performed using two-way analysis of variance (ANOVA) using GraphPad Prism.9.5.1 software. The p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Tensile strength
The ultimate tensile strength (UTS), failure strain percentage, the modulus of elasticity (E), and water content are shown in Figure 1. The UTS of the tendon form the contralateral side was higher on both the injury model (group II) animals and the repair model (group III) animals (Figure 1A). These values were higher than injury side (injury model and repair model) and the control swines, but were not statistically significant. The tendons of the contralateral side had significantly higher elastic modulus than the repair model (Group III) animals. These values were also higher than injury side (injury model and repair model) and the control swines, but were not statistically significant (Figure 1B). The modulus of elasticity of the tendons on the injury side of the repair animals were lower than the control group of animals. The tendons on the injury side of the repair model (group III) had significantly higher failure strain percentage than the contralateral side and injured tendons (group II). Whereas the failure strain percentage of the uninjured tendons of the contralateral side was significantly lower than the control group of animals (Figure 1C).
Figure 1:
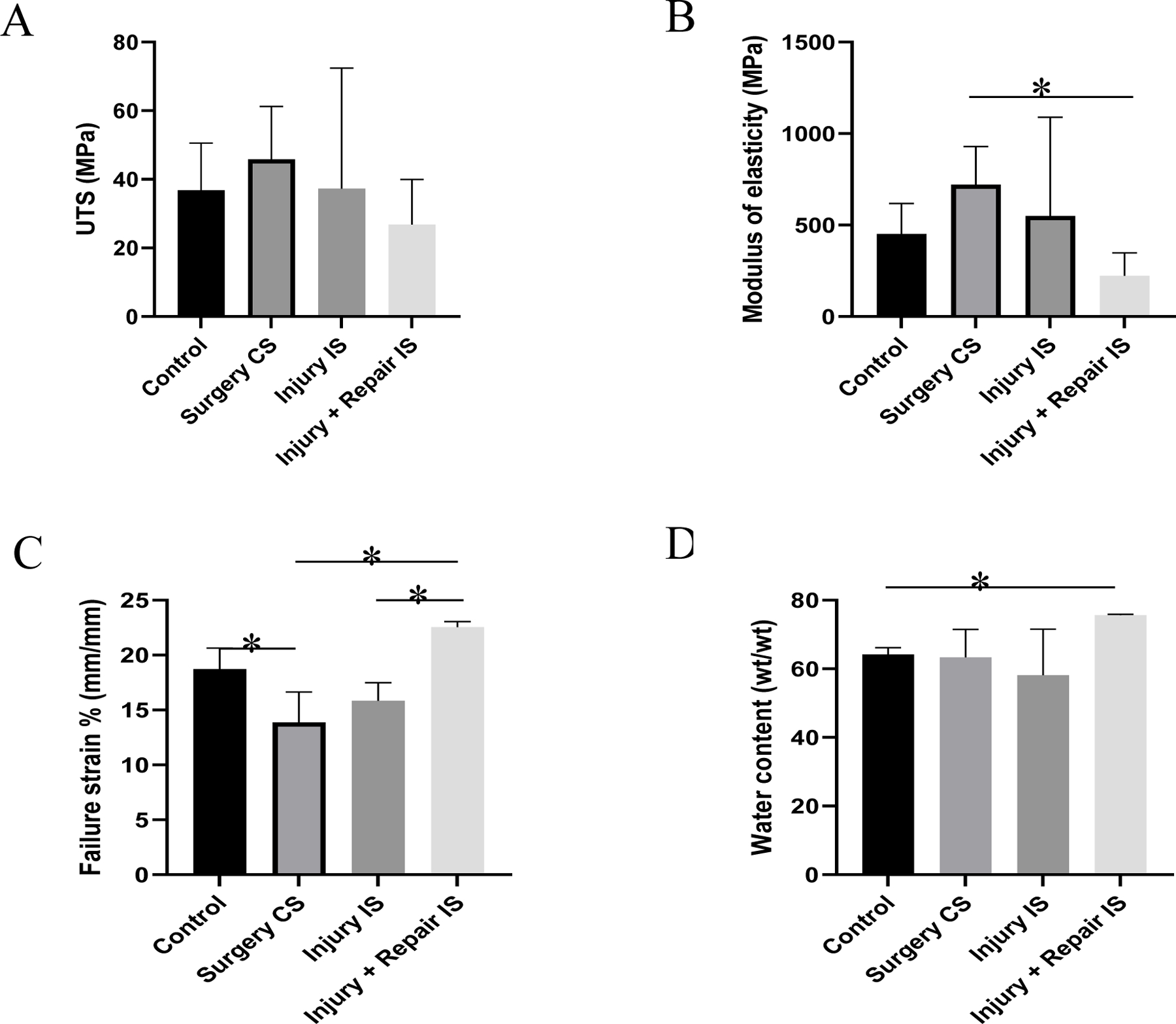
Mechanical properties and water content of tendon tissues. (A) Ultimate tensile strength, (B) Modulus of elasticity, (C) % Failure strain, (D) Water content. Control indicates the group of swine that received normal diet and no surgery. Surgery CS indicates uninjured tendon from contralateral side of swine that underwent either injury or injury + repair surgery. Injury IS indicating tendon from injury side that underwent injury. Injury + Repair IS indicating tendons from injury of swine that underwent Injury + Repair surgery. * Indicates significant difference (p<0.05, student-t test).
3.2. Water content
The water content of the tendon tissues is shown in Figure 1D. The injury side tendons of the repair model (group III) animals had the highest water content among the tested tendons which is significantly higher than the control group of animals. No significant difference in water content was observed among the other groups.
3.3. Dynamic mechanical analysis
The viscoelastic properties recorded at low frequencies are shown in Figure 2. The dynamic modulus and storage modulus increased with increase in frequency at the tested low frequencies while the loss modulus and the damping ability reduced with increasing frequencies. The dynamic modulus and storage modulus of the tendons on the contralateral side of the injury model (group II) and repair model (group III), were higher than control group of swines but were not statistically significant (Figure 2A,2B). The loss modulus and damping ability (Tan δ) did not show any significant difference between groups (Figure 2C,2D). The viscoelastic properties recorded at higher frequencies are shown in Figure 3. The dynamic modulus and storage modulus increased with the increase in frequency for all the tested tendons (Figure 3A,3B). Loss modulus and damping ability decreased initially at low frequencies and increased with increase in frequencies (Figure 3C,3D). The differences in viscoelastic properties at the respective frequencies were not statistically significant.
Figure 2:
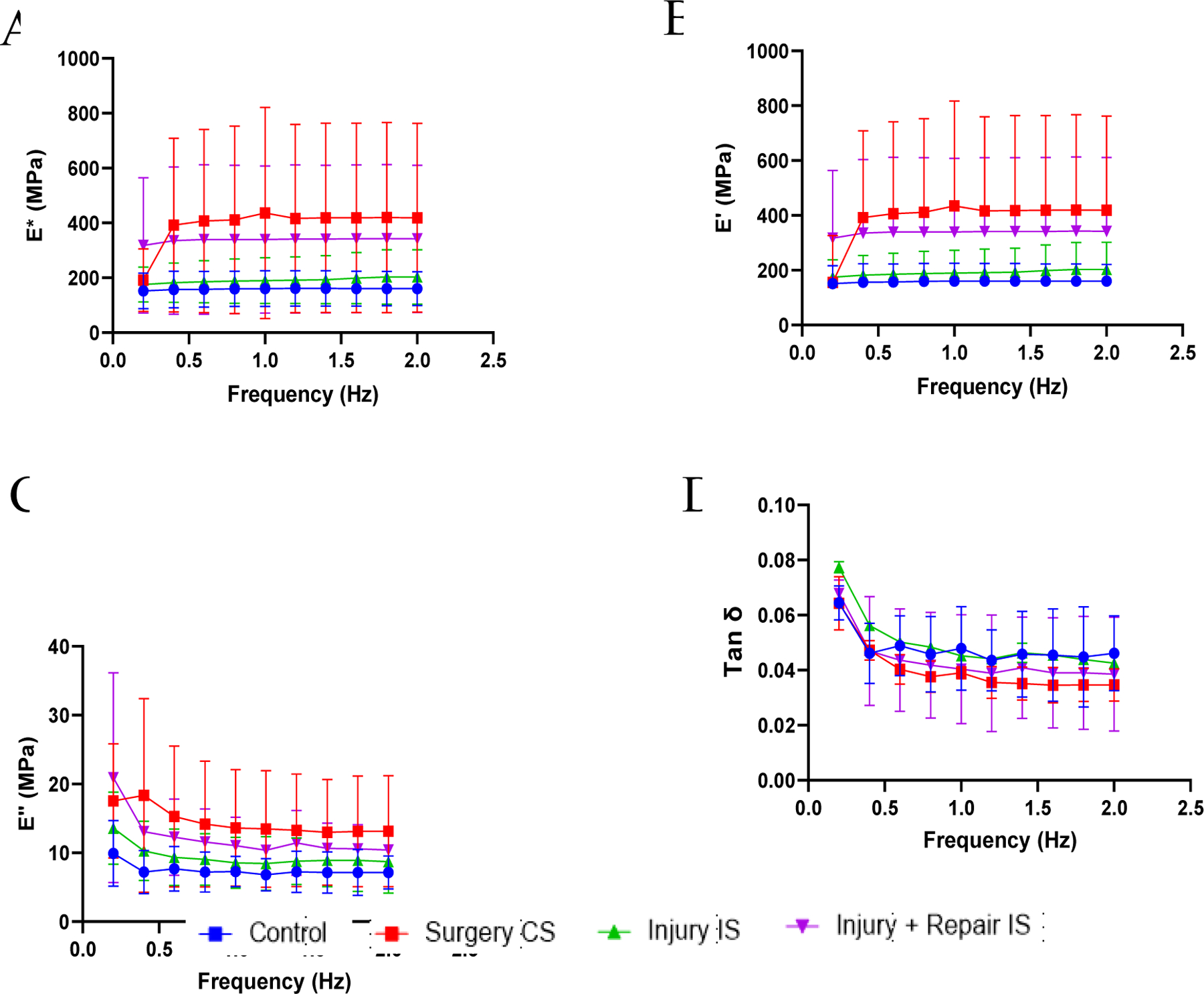
Viscoelastic properties of the tendon tissues at lower frequencies. (A) dynamic modulus (E*), (B) storage modulus (E’), (C) loss modulus (E”), and (D) damping ability (Tan δ). Control indicates the group of swine that received normal diet and no surgery. Surgery CS indicates uninjured tendon from contralateral side of swine that underwent either injury or injury + repair surgery. Injury IS indicating tendon from injury side that underwent injury. Injury + Repair IS indicating tendons from injury of swine that underwent Injury + Repair surgery.
Figure 3:
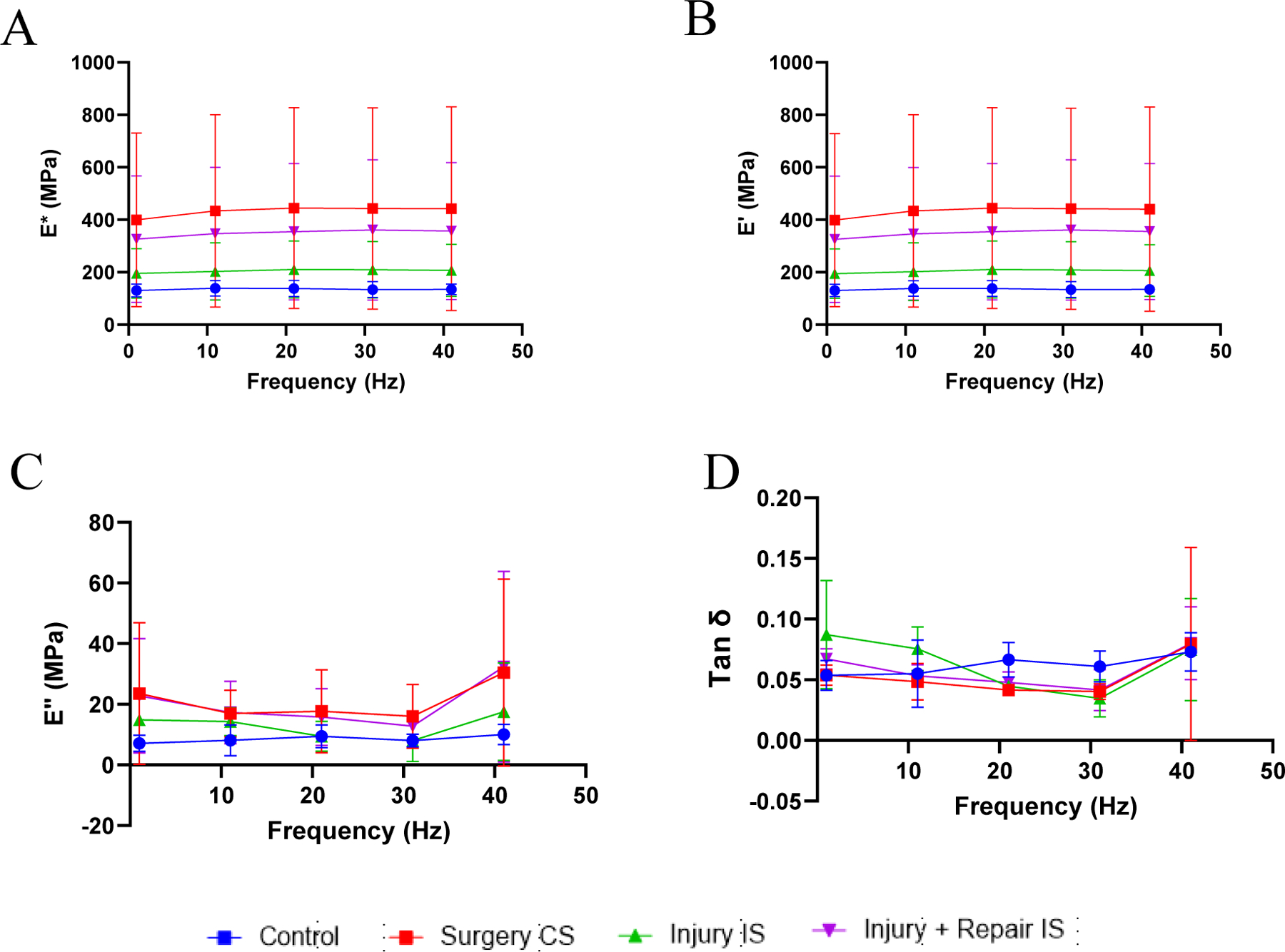
Viscoelastic properties of the tendon tissues at higher frequencies. (A) dynamic modulus (E*), (B) storage modulus (E’), (C) loss modulus (E”) and (D) damping ability (Tan δ). Control indicates the group of swine that received normal diet and no surgery. Surgery CS indicates uninjured tendon from contralateral side of swine that underwent either injury or injury + repair surgery. Injury IS indicating tendon from injury side that underwent injury. Injury + Repair IS indicating tendons from injury of swine that underwent Injury + Repair surgery.
3.4. Histology
The representative images of H&E staining of the tendon tissue are shown in Figure 4. The tendons of the control group had cells with elongated nucleus, tenocytes among the longitudinally arranged collagen fibers and extracellular matrix (ECM) was observed to be intact. The hyperlipidemic (group II and group III) animals were presented with the presence of fat infiltration, inflammatory cells, and actively dividing cells with rounded nucleus. We could observe clusters of inflammatory and dividing cells on the injury side of the repair model (group III) than the contralateral side of the repair model. The injury model (group II) had presented with less inflammatory cells in the injury side than the contralateral side but had fatty infiltration. Even though there were inflammatory cells and fatty infiltration, the tendons of the contralateral side had a better ECM organization than the injury side which was similar to the control group.
Figure 4:
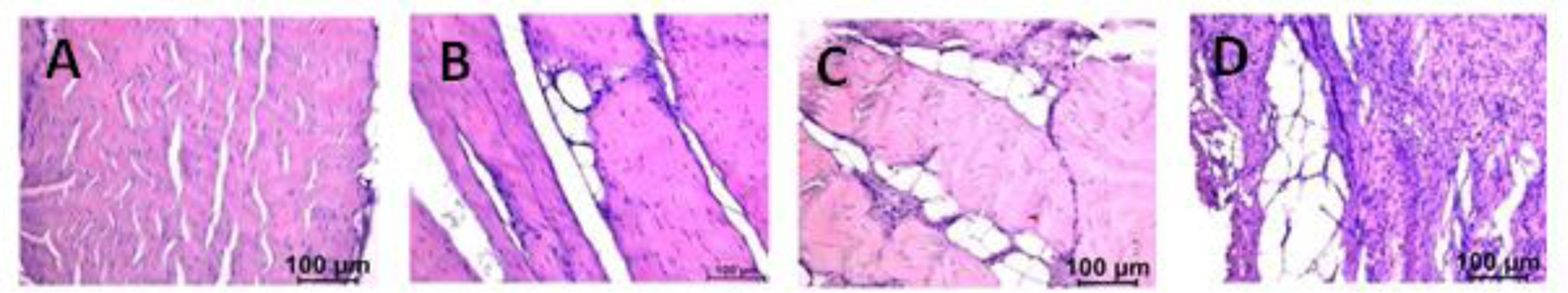
Representative image of tendon histology (H&E). (A) Control, (B) Surgery CS, (C) Injury IS, (D) Injury + Repair IS. Control indicates the group of swine that received normal diet and no surgery. Surgery CS indicates uninjured tendon from contralateral side of swine that underwent either injury or injury + repair surgery. Injury IS indicating tendon from injury side that underwent injury. Injury + Repair IS indicating tendons from injury of swine that underwent Injury + Repair surgery The images were acquired in 20x magnification.
4. Discussion
Hyperlipidemia is associated with depositing lipids in between the tendons by intercalating between the collagen fibers [2], and reduces the healing of rotator cuff injuries [4,5]. We created rotator cuff injury in swine (injury model) and injury plus repair in swine (repair model) and analyzed the biomechanical properties of the tendons 8 weeks post-surgery. The tendons of the injury side of the repair model had lower UTS and modulus of elasticity and higher failure percent strain indicating the impaired function and physiologically weak tendon than the tendon of contralateral side or the control swine. These values were in concurrence with previous studies [17]. The decrease in the modulus of elasticity in the repair model shows that the tendons have become more elastic. Mechanical properties vary with the location of tendon tissue. For instance, UTS of human Achilles tendon is 2258 ± 507 N and for patellar tendon is 5000N [18]. We have chosen the same tendon site, the infraspinatus tendon at the insertion to the grater tuberosity for the injury model and the repair model. We observed a dense fibrous tissue formed at the insertion and around the tendons in the injury side of the repair model. These tissues thicken and form a scar tissue that never regain the biomechanical properties even after many years [19]. The presence of actively dividing and inflammatory cells indicates that the tendon is undergoing remodeling. The tendons of the hyperlipidemic animals had fatty infiltration and disorganized ECM which is in concurrence with our earlier studies. Injury, fatty infiltration, and remodeling tendons have active recruitment of dividing cells and inflammatory cells [4,11,20–22].
The tendons of the contralateral side in the injury model and repair model had less fatty infiltration and had better organized ECM than the injury side. This could be because the tendons on the contralateral side being subjected to more loads than normal that mimics the dynamic loading regimes [19]. Meanwhile the presence of fibrous tissues would hinder the transfer of loads to the tendons and eventually act as a large tissue mass and the force transferred through the tendon tissue would have been reduced to a large extent. The tendons respond in accordance with loading regimes. In a previous study on rat models the supraspinatus tendons had shown increase in tensile strength and elastic modulus with increase in the loading regimes but had lowered tensile strength with low loading regimes [23].
In our study, the collagen fibers infiltrated with fat and fibrous tissues would have further weakened the inter-collagen bonds that are responsible for the tensile strength and elastic modulus by the aid of collagen fiber sliding to each other. Moreover, it would increase the cross-sectional area of load transfer and effectively reduce the load on the tendons. It is understood that the modulus is calculated from the linear portion of the stress-strain curve where the inter molecular sliding is the major force contributing the stiffness of the tendons [15,16]. This phenomenon is expected in fatty infiltration, but as the age increases the inter-collagen bonds which are divalent mature to form trivalent bonds and stabilizes the mechanical properties [24–27].
The viscoelastic properties, tensile strength, and modulus of elasticity of the tendons on the contralateral side showed higher values than the control tissues. Post surgeries the contralateral side are subjected to higher loads due to the weakening of the tendons on the injury side (injury model and repair model) and this characteristic is similar to the chronic adaptation of the tendons to loading regimes [23]. This could be the reason for increased mechanical properties of the tendons in the contralateral side. On the other hand, the increased water content could be a contributing factor in the decrease in modulus of elasticity of the repair tendon [14]. This increase in water content could be due to the fibrous tissues and the disorganized ECM complex. The viscoelastic properties of soft tissues increase with decrease in water content of the tissues [14]. We collected the tendon tissues from approximately 38 weeks old swine and hence we could rule out the effect of age on decreased biomechanical properties.
The biomechanical properties decrease with increase in age [28–30]. Our study showed the decrease in biomechanical properties on the injury side of the infraspinatus tendon of hyperlipidemic swine compared to the normal swine and an increase in the biomechanical properties in the infraspinatus tendons of the contralateral side of the injury. Further investigation is warranted with statistically significant number of animals, longer time duration, and analysis of injury models on swine with normal diet, which would reveal the relationship between hyperlipidemia, healing of injury, and the changes in ex-vivo biomechanical properties. This would give deeper understanding and help in developing new avenues of treatment approaches and biomaterial development.
5. Limitations
In this pilot study, we have only two animals in each of the injury and repair. The mechanical properties vary with variation in physical activities and subject to individual variation. The measurement of extension was calculated from the crosshead displacement of clamps to compute the strain values. Measurement with video extensometer would have been more accurate in the analysis. Additional studies in a larger sample size of experimental animals and longer time duration are required to get deeper understanding between the biomechanical properties and hyperlipidemia following tendon injury and repair.
6. Conclusion
This study provided the pilot data on changes in the biomechanical properties of the infraspinatus tendons in hyperlipidemic swine that underwent injury and repair. Hyperlipidemia and fibrous tissue around the injury site could have been the factor for decreased modulus of elasticity and viscoelastic properties in swine on the injury side. However, long-term studies with larger sample size of experimental animals are required.
Funding:
The research work of DKA is supported by the R01 HL144125 and R01 HL147662 grants from the National Institutes of Health, USA. The content of this critical review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interest: All the authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication: All the authors have read the manuscript and consented for publication.
References
- 1.Agrawal D, Dougherty K, Dilisio M. Vitamin D and the immunomodulation of rotator cuff injury. J Inflamm Res 9 (2016): 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Qu J. The effects of hyperlipidemia on rotator cuff diseases: a systematic review. J Orthop Surg Res 13 (2018): 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minagawa H, Yamamoto N, Abe H, et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J Orthop 10 (2013): 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang WH, Bonavida V, Agrawal DK, et al. Hyperlipidemia in tendon injury: chronicles of low-density lipoproteins. Cell Tissue Res 392 (2023): 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdani AN, Rai V, K Agrawal D. Rotator Cuff Health, Pathology, and Repair in the Perspective of Hyperlipidemia. Journal of Orthopaedics and Sports Medicine 4 (2022): 163–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundi S, Massaro M, Scoditti E, et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res 114 (2018): 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgio V, Civera M, Reinoso MR, et al. Mechanical Properties of Animal Tendons: A Review and Comparative Study for the Identification of the Most Suitable Human Tendon Surrogates. Processes 10 (2022): 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabrowska S, Grabowski K, Mlyniec A. Rehydration of the Tendon Fascicle Bundles Using Simulated Body Fluid Ensures Stable Mechanical Properties of the Samples. Materials 15 (2022): 3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin TTL, Lin CH, Chang CL, et al. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med 43 (2015): 2126–2132. [DOI] [PubMed] [Google Scholar]
- 10.Thankam FG, Wilson VED, Agrawal DK. Animal models of inflammatory musculoskeletal diseases for tissue engineering and regenerative medicine: updates and translational application. Advances in Animal Experimentation and Modeling Elsevier; (2022): 123–135. [Google Scholar]
- 11.Fang W, Sekhon S, Teramoto D, et al. Pathological alterations in the expression status of rotator cuff tendon matrix components in hyperlipidemia. Mol Cell Biochem 478 (2023): 1887–1898. [DOI] [PubMed] [Google Scholar]
- 12.Thankam FG, Wilson VED, Radwan MM, et al. Involvement of ischemia-driven 5- lipoxygenase-resolvin-E1-chemokine like receptor-1 axis in the resolution of post- coronary artery bypass graft inflammation in coronary arteries. Mol Biol Rep 49 (2022): 3123–3134. [DOI] [PubMed] [Google Scholar]
- 13.Mlyniec A, Dabrowska S, Heljak M, et al. The dispersion of viscoelastic properties of fascicle bundles within the tendon results from the presence of interfascicular matrix and flow of body fluids. Materials Science and Engineering: C 130 (2021): 112435. [DOI] [PubMed] [Google Scholar]
- 14.Edwards JH, Ingham E, Herbert A. Decellularisation affects the strain rate dependent and dynamic mechanical properties of a xenogeneic tendon intended for anterior cruciate ligament replacement. J Mech Behav Biomed Mater 91 (2019): 18–23. [DOI] [PubMed] [Google Scholar]
- 15.Chandrashekar N, Hashemi J, Slauterbeck J, et al. Low-load behaviour of the patellar tendon graft and its relevance to the biomechanics of the reconstructed knee. Clinical Biomechanics 23 (2008): 918–925. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhury S, Holland C, Thompson MS, et al. Tensile and shear mechanical properties of rotator cuff repair patches. J Shoulder Elbow Surg 21 (2012): 1168–1176. [DOI] [PubMed] [Google Scholar]
- 17.Halder A, Zobitz ME, Schultz F, et al. Mechanical properties of the posterior rotator cuff. Clinical Biomechanics 15 (2000): 456–462. [DOI] [PubMed] [Google Scholar]
- 18.Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol 108 (2010): 670–675. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita H, Ochi M, Ikuta Y. Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Arch Orthop Trauma Surg 116 (1997): 454–462. [DOI] [PubMed] [Google Scholar]
- 20.Thankam FG, Diaz C, Chandra I, et al. Hybrid interpenetrating hydrogel network favoring the bidirectional migration of tenocytes for rotator cuff tendon regeneration. J Biomed Mater Res B Appl Biomater 110 (2022): 467–477. [DOI] [PubMed] [Google Scholar]
- 21.Thankam FG, Agrawal DK. Hypoxia-driven secretion of extracellular matrix proteins in the exosomes reflects the asymptomatic pathology of rotator cuff tendinopathies. Can J Physiol Pharmacol 99 (2021): 224–230. [DOI] [PubMed] [Google Scholar]
- 22.Rai V, Dietz NE, Dilisio MF, et al. Vitamin D attenuates inflammation, fatty infiltration, and cartilage loss in the knee of hyperlipidemic microswine. Arthritis Res Ther 18 (2016): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rooney SI, Torino DJ, Baskin R, et al. Rat supraspinatus tendon responds acutely and chronically to exercise. J Appl Physiol (1985) 123 (2017): 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Depalle B, Qin Z, Shefelbine SJ, et al. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater 52 (2015): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couppé C, Hansen P, Kongsgaard M, et al. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107 (2009): 880–886. [DOI] [PubMed] [Google Scholar]
- 26.Singh D, Rai V, K Agrawal D. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol Cardiovasc Med 7 (2023): 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohindra R, Mohindra R, Agrawal DK, et al. Bioactive extracellular matrix fragments in tendon repair. Cell Tissue Res 390 (2022): 131–140. [DOI] [PubMed] [Google Scholar]
- 28.Codding JL, Keener JD. Natural History of Degenerative Rotator Cuff Tears. Curr Rev Musculoskelet Med 11 (2018): 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorpe CT, Godinho MSC, Riley GP, et al. The interfascicular matrix enables fascicle sliding and recovery in tendon and behaves more elastically in energy storing tendons. J Mech Behav Biomed Mater 52 (2015): 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder A, Zobitz ME, Schultz F, et al. Mechanical properties of the posterior rotator cuff. Clinical Biomechanics 15 (2000): 456–462. [DOI] [PubMed] [Google Scholar]


