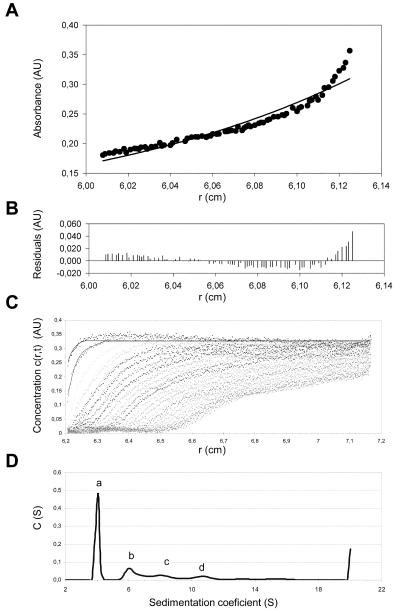
FIG. 2.
Association states of TEV hisHC-Pro in solution. Sedimentation equilibrium at 10,000 rpm (Beckman Optima XL-A; An50Ti rotor) and 4°C of a sample containing TEV hisHC-Pro at a 1.3-mg/ml concentration (panels A and B) shows that the purified protein in solution is present in more than one form. (A) Sedimentation equilibrium data (dots) and fit corresponding to one homogeneous component (solid line). (B) Residuals between estimated values and experimental data for the one-component fit. Sedimentation velocity data (panels C and D) obtained with a 0.3-mg/ml sample of TEV hisHC-Pro subjected to a 50,000 rpm run at 12°C are shown. (C) Concentration profiles where dotted lines represent the experimental data and solid lines represent the fittings performed by application of the SEDFIT program. (D) Concentration distribution versus sedimentation coefficient showing four different association states (peaks a, b, c, and d), compatible with the dimers, tetramers, hexamers, and octamers of the protein.