Abstract
Background
Associations of coronary heart disease (CHD) with plasma lipids are well described, but the associations with characteristics of lipoproteins (which transport lipids) remain unclear.
Methods and Results
UK Biobank is a prospective study of 0.5 million adults. Analyses were restricted to 89 422 participants with plasma lipoprotein and apolipoprotein measures from Nightingale nuclear magnetic resonance spectroscopy and without CHD at baseline. CHD risk was positively associated with concentrations of very‐low‐density lipoproteins, intermediate‐density lipoproteins, and low‐density lipoproteins (LDL), and inversely associated with high‐density lipoproteins. Hazard ratios (99% CIs) per SD were 1.22 (1.17–1.28), 1.16 (1.11–1.21), 1.20 (1.15–1.25), and 0.90 (0.86–0.95), respectively. Larger subclasses of very‐low‐density lipoproteins were less strongly associated with CHD risk, but associations did not materially vary by size of LDL or high‐density lipoprotein. Given lipoprotein particle concentrations, lipid composition (including cholesterol) was not strongly related to CHD risk, except for triglyceride in LDL particles. Apolipoprotein B was highly correlated with LDL concentration (r=0.99), but after adjustment for apolipoprotein B, concentrations of very‐low‐density lipoprotein and high‐density lipoprotein particles remained strongly related to CHD risk.
Conclusions
This large‐scale study reliably quantifies the associations of nuclear magnetic resonance–defined lipoprotein characteristics with CHD risk. CHD risk was most strongly related to particle concentrations, and separate measurements of lipoprotein concentrations may be of greater value than the measurement by apolipoprotein B, which was largely determined by LDL concentration alone. Furthermore, there was strong evidence of positive association with mean triglyceride molecules per LDL particle but little evidence of associations with total triglycerides or other lipid and lipoprotein fractions after accounting for lipoprotein concentrations.
Keywords: apolipoproteins, cholesterol, coronary heart disease, lipoproteins, nuclear magnetic resonance, triglycerides, UK biobank
Subject Categories: Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- IDL
intermediate‐density lipoprotein
- NMR
nuclear magnetic resonance
Clinical Perspective.
What Is New?
This is one of few large‐scale studies to assess the separate associations of lipoprotein particle concentration, size, and composition with coronary heart disease risk.
Separate measurements of lipoprotein concentrations may be of greater value than the single measurement by apolipoprotein B, which was largely determined by low‐density lipoprotein concentration alone.
The study indicates that the associations of plasma lipids with coronary heart disease risk are likely to be accounted for largely by lipoprotein particle concentrations; however, after adjusting for lipoprotein particle concentration, mean triglyceride molecules per low‐density lipoprotein particle remained positively related to coronary heart disease risk.
What Are the Clinical Implications?
Nightingale nuclear magnetic resonance provides a wealth of information about lipoprotein particles, including their concentration, size, and content of lipid, although the practicalities of performing this new method in a typical clinical setting, including logistics and cost‐effectiveness, remain to be explored.
Furthermore, the study suggests that, in addition to lipoprotein concentrations, mean triglyceride molecules per low‐density lipoprotein particles may be a valuable additional biomarker of coronary heart disease risk.
Lipoproteins are complex particles that transport lipids in the circulation. Although the associations of plasma lipids with coronary heart disease (CHD) risk are well described, 1 , 2 , 3 more recent evidence suggests these associations may be driven more directly by the total circulating concentration of apolipoprotein B (ApoB)–containing lipoprotein particles. 1 , 4 Although total ApoB levels strongly predict CHD risk, ApoB‐containing particles differ substantially by class. Very‐low‐density lipoprotein (VLDL), intermediate‐density lipoprotein (IDL), and low‐density lipoprotein (LDL) vary between and within class by size and lipid composition. 5 Despite considerable research in this area, the relevance of these different lipoprotein characteristics to CHD risk remains unclear.
Previous studies comparing the strength of associations between lipids and ApoB‐containing lipoproteins relied on total ApoB levels as an indirect measure of the total concentration of all ApoB‐containing particles (each VLDL, IDL, and LDL particle has a single ApoB molecule). 1 , 4 However, VLDL and IDL are present in the plasma at much lower concentrations than LDL, and thus variations in VLDL, IDL, and LDL particle concentration may be obscured by measurements of total ApoB concentration alone. Furthermore, as traditional lipid measures are dependent on both the concentration of lipoproteins and their composition (size and lipid content), the atherogenic potential of lipoprotein may well be dependent on both these characteristics, but most previous studies have not described lipid composition for each of the different lipoprotein classes.
Nuclear magnetic resonance (NMR) spectroscopy is a high‐throughput technology for metabolic profiling. Compared with the previous NMR method, 6 , 7 the current Nightingale NMR platform has the strength of providing details on many characteristics of major classes and subclasses of lipoproteins 5 and shows high consistency with the same lipid and apolipoprotein biomarkers measured using standard clinical chemistry method. 8 , 9 To the authors' knowledge, this study based on 89 422 participants in UK Biobank is the largest study with blood metabolites measured by the Nightingale NMR spectroscopy. The study aims to reliably quantify the associations of CHD risk with concentration of each of the major lipoprotein particle classes and to examine the effects of lipoprotein size and lipid composition after accounting for particle concentration.
Methods
Study Design and Participants
Details of the UK Biobank study design and data collection methods have been reported previously. 10 In brief, it is a prospective cohort study of ≈500 000 adults recruited from the general population of the United Kingdom from 2006 to 2010. At recruitment, participants completed questionnaires, had physical measurements taken, and biological samples were collected (including blood for long‐term storage). A resurvey of the full baseline assessment was conducted on a subset of 20 346 participants who lived within a 35‐km radius of the assessment center at Stockport, with an average of 4.2 years after the first sampling, of which a random subset of blood samples were selected for measuring by Nightingale NMR spectroscopy. Ethical approval was obtained by the North West Research Ethics Committee. All participants in the UK Biobank provided informed written consent to take part in the study. Data from the UK Biobank are available to researchers after registration at the UK Biobank server. The data cleaning and coding used to generate the findings of this study are available from the corresponding author upon reasonable request.
Measurement of Biomarkers
The Nightingale NMR spectroscopy (Nightingale Health, Finland) was used for metabolic profiling of the baseline plasma samples of 121 540 participants (a random subset of the initial cohort) and 5306 blood samples taken at resurvey. The following measurements of lipoproteins and lipids were available for analyses: the concentration of lipoproteins (including VLDL, IDL, LDL, and high‐density lipoprotein [HDL]) and 13 subclasses of these lipoproteins, as defined by particle size; the average diameter of lipoprotein; the total lipid concentration (including cholesteryl esters, free cholesterol, phospholipids, and triglycerides) of lipoprotein; and the mean lipid composition of lipoprotein (calculated by dividing the lipid concentration by the corresponding lipoprotein particle concentration); and the concentration of apolipoprotein A1 and ApoB 5 (Table S1).
Case Definition of CHD
Incident CHD was defined as the first‐ever myocardial infarction, unstable angina pectoris, coronary‐related procedure, or coronary‐related death. Events were identified from hospital episode statistics and from the Office for National Statistics cause of death data (Table S2). 11
Statistical Analysis
The analyses excluded participants with prior CHD or those taking statins at baseline (Figure S1). Cox proportional hazards models, stratified by sex and age, and adjusted for education, region, Townsend Deprivation Index, smoking, alcohol intake, and body mass index, were used to calculate hazard ratios (HRs) for the associations of lipoprotein characteristics with incident CHD. Given the multiple number of HRs presented, we lowered the significance threshold to 0.01 and, thus, 99% CIs were calculated. To examine the separate effect of these characteristics on CHD risk, analyses of the size and composition of lipoproteins were further adjusted for lipoprotein concentrations of VLDL, LDL, and HDL (IDL concentration was not included for further adjustment because of its high correlation with LDL).
HRs were corrected for regression dilution bias (ie, categorizing people by their baseline concentrations and estimating the long‐term average concentration in each category using the correlation between resurvey and baseline measurements) and therefore describe associations of usual lipoprotein levels with CHD risk. 12 “Usual” levels in the plot were estimated from the mean value at resurvey within each baseline defined group, representing an unbiased estimate of the long‐term average level in each baseline‐defined group. The SD of the usual values was obtained by multiplying the baseline SD by the square root of the regression dilution ratio, and CIs in the plots were calculated using the variance of the log risk, which appropriately attributes variance to all groups, including the reference. 13
The change in the log likelihood ratio χ2 between models with and without the lipoprotein parameter is a measure of extent of variance explained by the lipoprotein parameter in addition to the other variables in the model. This statistic provides not only a significance test for the improvement in fit from including the main lipoprotein term but also a quantitative measure of the extent to which the lipoprotein term improves risk prediction in different models (eg, with and without adjustment for confounders or other lipoprotein characteristics). 14 Discordance analysis (used to deal with the challenge of discriminating among highly correlated markers) was also used to judge which biomarker is more responsible for risk among highly correlated lipids or lipoproteins by comparing the HRs among the participants who are discordant in 2 highly correlated biomarkers. 15 , 16
Sensitivity analysis of the main associations were conducted by excluding CHD events in the first 2 years of follow‐up and by further adjusting for other potential confounders, including waist circumference, systolic blood pressure, diabetes, and chronic kidney disease, at baseline, fasting time, and spectrometer. The associations of apolipoprotein A1 and ApoB concentrations with incidence CHD, as well as the associations of lipids and apolipoproteins measured using either clinical chemistry or Nightingale NMR method, were estimated for comparison. All the measures in the tables and figures, except the ones we noted as “measured by clinical chemistry,” were measured by Nightingale NMR. All analyses were conducted with SAS version 9.4, and all figures were generated in R version 4.0.1.
Results
After exclusions, 89 422 participants remained, of which 3821 had an incident CHD event during a mean follow‐up of 11.5 years. Compared with participants who did not have an incident CHD event, those with incident CHD were on average slightly older, more likely to be men and current smokers, have lower education, and have slightly higher body mass index and systolic blood pressure (Table 1). The baseline characteristics of the study population showed no difference with the whole UK Biobank participants (Table S3).
Table 1.
Baseline Characteristics in Study Population by Incident CHD
| Characteristic | Incident CHD | ||
|---|---|---|---|
| No | Yes | All | |
| No. of participants | 85 601 | 3821 | 89 422 |
| Sociodemographic factors | |||
| Baseline age, y | 55.0 (8.0) | 59.2 (7.1) | 55.2 (8.0) |
| Male sex, % | 41.8 | 64.5 | 42.8 |
| White race, % | 95.0 | 95.5 | 95.0 |
| University education, % | 40.8 | 31.6 | 40.4 |
| Townsend Deprivation Index* | −1.4 (3.0) | −1.2 (3.2) | −1.4 (3.0) |
| Lifestyle factors, % | |||
| Current smoker | 10.1 | 16.6 | 10.5 |
| Current regular alcohol drinker | 70.7 | 68.3 | 70.6 |
| Anthropometry | |||
| Body mass index, kg/m2 | 26.9 (4.6) | 27.9 (4.6) | 27.0 (4.6) |
| Waist circumference, cm | 88.5 (12.9) | 93.9 (12.5) | 88.7 (12.9) |
| Waist/hip ratio | 0.86 (0.09) | 0.91 (0.08) | 0.86 (0.09) |
| Lipids and apolipoproteins measured by clinical chemistry | |||
| LDL cholesterol, mmol/L | 3.7 (0.8) | 3.9 (0.8) | 3.7 (0.8) |
| HDL cholesterol, mmol/L | 1.5 (0.4) | 1.3 (0.3) | 1.5 (0.4) |
| Total triglycerides, mmol/L | 1.7 (1.0) | 2.0 (1.1) | 1.7 (1.0) |
| Apolipoprotein A‐I, g/L | 1.5 (0.3) | 1.5 (0.3) | 1.5 (0.3) |
| Apolipoprotein B, g/L | 1.1 (0.2) | 1.1 (0.2) | 1.1 (0.2) |
| Blood pressure and diabetes | |||
| Systolic blood pressure, mm Hg | 136.3 (18.4) | 144.8 (19.0) | 136.7 (18.5) |
| Baseline diabetes, %† | 2.0 | 4.5 | 2.1 |
| Fasting time, h | 3.0 (2.0) | 3.0 (1.0) | 3.0 (2.0) |
Baseline characteristics of those with and those without incident CHD during follow‐up among 89 422 participants. Analyses exclude those with missing or outlying values for metabolites or key covariates and those with prior CHD or taking statins at baseline. Continuous variables are presented as mean (SD) (except for skew variable fasting time, presented as median [quartile range]), and categorical variables are presented as column percentages. CHD indicates coronary heart disease; HDL, high‐density lipoprotein; and LDL, low‐density lipoprotein.
Area‐level measure of material deprivation.
The low diabetes prevalence is attributable to the exclusion of individuals with baseline cardiovascular disease, which had large overlap with the individuals with diabetes at baseline. Before excluding baseline cardiovascular disease, the prevalence of diabetes in the study population is 6% (the prevalence is also 6% for the whole UK Biobank participants).
There were 1065 participants (after exclusions) with lipoprotein measures both at baseline and resurvey (measured on average 4.2 years after baseline assessment) (Table S3). The baseline levels of lipids and apolipoproteins (measured by clinical chemistry) among the participants with repeated measures were similar with the total study population, although there were some differences in the baseline socioeconomic and lifestyle factors (Table S3). The mean levels of lipoprotein measures among resurveyed participants were also not statistically different between baseline and resurvey (Table S4). The regression dilution ratios between baseline and resurvey of most measures of lipoprotein characteristics ranged from 0.5 to 0.8, although this was slightly lower for LDL particle diameter (0.28) and mean cholesteryl ester molecules per LDL particle (0.42) (Table S5); further adjustment for the differences in baseline characteristics between resurveyed and study population did not materially change the estimates of regression dilution ratios.
CHD risk was positively associated with usual VLDL, IDL, and LDL particle concentrations and inversely associated with HDL concentration (Figure 1). After adjusting for confounders and correcting for regression dilution, HRs (99% CIs) per usual SD higher level of VLDL, IDL, LDL, and HDL particle concentration were 1.22 (1.17–1.28), 1.16 (1.11–1.21), 1.20 (1.15–1.25), and 0.90 (0.86–0.95), respectively. With the same adjustment, the CI of the HR of ApoB concentration (1.20 [1.15–1.25]) overlapped the HR of LDL particle concentration, and the overlap was also between HR of apolipoprotein A1 (0.88 [0.83–0.92]) and HDL concentration (Table S6).
Figure 1. Lipoprotein particle concentration vs coronary heart disease risk.
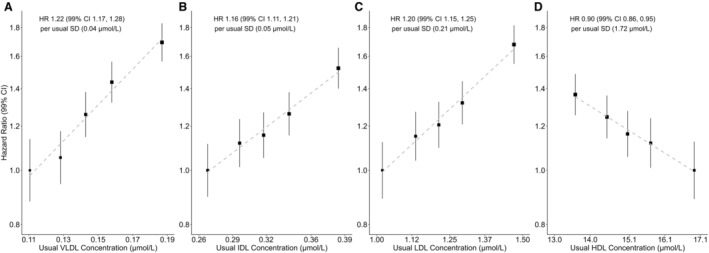
Hazard ratios (HRs) per usual SD higher level of particle concentration among 89 422 participants (stratified by quintiles). A, Very‐low‐density lipoprotein (VLDL) particle concentration. B, Intermediate‐density lipoprotein (IDL) particle concentration. C, Low‐density lipoprotein (LDL) particle concentration. D, High‐density lipoprotein (HDL) particle concentration. HRs calculated by Cox proportional hazards models, stratified by age and sex, and adjusted for ethnicity, education, region, Townsend Deprivation Index, smoking, alcohol, and body mass index. “Usual” levels are estimated from the mean value at resurvey within each baseline‐defined group, providing an unbiased estimate of the long‐term average level in each baseline‐defined group. “Usual SD” was obtained from the baseline SD through multiplication by square root of the regression dilution ratio. Area of the square is inversely proportional to the variance of the category‐specific log risk. CI indicates confidence interval; HDL, high‐density lipoproteins; HR, hazard ratio; IDL, intermediate‐density lipoproteins; LDL, low‐density lipoproteins; SD, standard deviation; and VLDL, very‐low‐density lipoproteins.
The strength of the associations of lipoprotein concentration and CHD risk varied across subclass of VLDL, with progressively stronger associations for smaller subclasses: HR was 1.22 (99% CI, 1.17–1.28) for the very small subclass of VLDL particles but 1.11 (99% CI, 1.07–1.16) for the extremely large subclass of VLDL (Table 2). By contrast, the strengths of the associations of lipoprotein concentration and CHD risk were similar and not statistically different across LDL subclasses. For HDL, there were slight variations in the associations by subclass but no evidence of increasing or decreasing trend in associations from small to very large HDL. The findings on lipoprotein subclass were consistent with analyses of lipoprotein particle diameter and CHD risk (Table 3 and Figure S2): there was no evidence of associations with either usual LDL diameter (1.04 [99% CI, 0.99–1.10]) or usual HDL diameter (0.96 [99% CI, 0.90–1.03]) after adjusting for lipoprotein concentrations but an inverse association with usual VLDL diameter (0.92 [99% CI, 0.86–0.99]).
Table 2.
Lipoprotein Particle Concentration Versus CHD Risk, by Particle Subclass
| Lipoprotein subclass | Particle concentration, μmol/L | A: adjusted for age and sex only | B: adjusted for age, sex, and other confounders | |||
|---|---|---|---|---|---|---|
| Baseline mean | Usual SD | HR (99% CI) | LR χ2 | HR (99% CI) | LR χ2 | |
| VLDL | ||||||
| Extremely large | 0.002 | 0.001 | 1.18 (1.14–1.22) | 121.4 | 1.11 (1.07–1.16) | 44.2 |
| Very large | 0.003 | 0.002 | 1.23 (1.18–1.28) | 180.7 | 1.16 (1.12–1.21) | 84.5 |
| Large | 0.010 | 0.004 | 1.24 (1.19–1.29) | 189.3 | 1.18 (1.13–1.23) | 95.9 |
| Medium | 0.036 | 0.009 | 1.25 (1.20–1.30) | 184.6 | 1.21 (1.16–1.26) | 126.0 |
| Small | 0.039 | 0.010 | 1.27 (1.22–1.32) | 225.1 | 1.22 (1.17–1.27) | 139.8 |
| Very small | 0.057 | 0.010 | 1.26 (1.21–1.31) | 198.7 | 1.22 (1.17–1.28) | 150.1 |
| All | 0.147 | 0.035 | 1.28 (1.23–1.33) | 230.1 | 1.22 (1.17–1.28) | 148.7 |
| IDL | 0.323 | 0.054 | 1.18 (1.13–1.23) | 97.4 | 1.16 (1.11–1.21) | 82.0 |
| LDL | ||||||
| Large | 0.754 | 0.128 | 1.21 (1.16–1.26) | 139.2 | 1.19 (1.14–1.24) | 119.1 |
| Medium | 0.301 | 0.054 | 1.20 (1.15–1.25) | 125.8 | 1.18 (1.13–1.23) | 94.8 |
| Small | 0.174 | 0.028 | 1.24 (1.19–1.29) | 170.7 | 1.20 (1.15–1.26) | 127.1 |
| All | 1.229 | 0.207 | 1.22 (1.17–1.27) | 148.3 | 1.20 (1.15–1.25) | 120.6 |
| HDL | ||||||
| Very large | 0.243 | 0.082 | 0.85 (0.80–0.89) | 70.7 | 0.94 (0.89–0.99) | 9.2 |
| Large | 1.451 | 0.672 | 0.75 (0.70–0.79) | 201.5 | 0.83 (0.78–0.88) | 71.6 |
| Medium | 3.837 | 0.696 | 0.80 (0.77–0.84) | 136.8 | 0.86 (0.82–0.90) | 62.2 |
| Small | 9.583 | 0.907 | 1.00 (0.95–1.04) | 0.1 | 0.99 (0.95–1.04) | 0.2 |
| All | 15.114 | 1.722 | 0.85 (0.81–0.89) | 80.2 | 0.90 (0.86–0.95) | 31.1 |
HRs per usual SD higher level of particle concentration among 89 422 participants. HRs calculated by Cox proportional hazards models with: (A) stratification by age and sex; and (B) model A with further adjustment for ethnicity, education, region, Townsend Deprivation Index, smoking, alcohol, and body mass index. LR χ2 improvement with the addition of the exposure variable to the model with stated adjustments. “Usual SD” was obtained from the baseline SD through multiplication by square root of the regression dilution ratio, representing an unbiased estimate of the long‐term average level in each baseline‐defined group. CHD indicates coronary heart disease; HDL, high‐density lipoprotein; HR, hazard ratio; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; LR, likelihood ratio; and VLDL, very‐low‐density lipoprotein.
Table 3.
Lipoprotein Particle Size Versus CHD Risk
| Lipoprotein type | Particle diameter, nm | A: adjusted for age and sex only | B: adjusted for age, sex, and other confounders | C: further adjusted for lipoprotein particle concentration | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline mean | Usual SD | HR (99% CI) | LR χ2 | HR (99% CI) | LR χ2 | HR (99% CI) | LR χ2 | |
| VLDL | 38.6 | 1.0 | 1.18 (1.13–1.24) | 90.8 | 1.10 (1.05–1.15) | 25.2 | 0.92 (0.86–0.99) | 9.3 |
| LDL | 23.9 | <0.1 | 0.99 (0.95–1.03) | 0.7 | 1.03 (0.98–1.07) | 2.4 | 1.04 (0.99–1.10) | 4.9 |
| HDL | ,9.7 | 0.2 | 0.76 (0.72–0.80) | 193.1 | 0.83 (0.79–0.88) | 70.5 | 0.96 (0.90–1.03) | 1.9 |
HRs per usual SD higher level of average particle diameter among 89 422 participants. HRs calculated by Cox proportional hazards models with: (A) stratification by age and sex; (B) model A with further adjustment for ethnicity, education, region, Townsend Deprivation Index, smoking, alcohol, and body mass index; and (C) model B with further adjustment for concentrations of VLDL, LDL, and HDL particles. LR χ2 improvement with the addition of the exposure variable to the model with stated adjustments. “Usual SD” was obtained from the baseline SD through multiplication by square root of the regression dilution ratio, representing an unbiased estimate of the long‐term average level in each baseline‐defined group. Particle diameter of intermediate‐density lipoprotein is unavailable in Nightingale nuclear magnetic resonance platform. CHD indicates coronary heart disease; HDL, high‐density lipoprotein; HR, hazard ratio; LDL, low‐density lipoprotein; LR, likelihood ratio; and VLDL, very‐low‐density lipoprotein.
Table 4 shows the associations of lipoprotein composition and CHD risk. In analyses of VLDL particle composition, free cholesterol was inversely associated with CHD risk (0.92 [99% CI, 0.86–0.99]) after adjustment for VLDL, LDL, and HDL particle concentrations, but the proportional reduction in the likelihood ratio χ2 statistic (from 118 to 9, a reduction of 92%) with progressive adjustments indicated the strong likelihood for residual confounding in this association. There was no evidence of associations with other lipid fractions in VLDL particle. For LDL particle composition, there was strong evidence of a positive association of CHD risk with mean triglyceride molecule (1.18 [99% CI, 1.10–1.26]; Figure 2) after adjusting for lipoprotein concentration but no evidence of associations with levels of cholesterol (1.03 [99% CI, 0.94–1.14]), free cholesterol (1.02 [99% CI, 0.92–1.14]), cholesteryl esters (1.03 [99% CI, 0.95–1.12]), or phospholipids (1.06 [99% CI, 0.98–1.14]) (Table 4 and Figure S3). There were similar findings for HDL particle composition, with no evidence of an association with lipid fractions except triglycerides (1.16 [99% CI, 1.03–1.31]); however, the proportional reduction in the likelihood ratio χ2 statistic (from 175 to 10, a reduction of 94%) with progressive adjustments indicated the strong likelihood for residual confounding in this association (Table 4). The analyses of IDL revealed similar findings to those of LDL, with only the mean triglyceride molecules per particle being associated with CHD risk after adjusting for particle concentrations, although the strength of the association was weaker (1.12 [99% CI, 1.05–1.19]) (Table 4 and Figure S4). Similarly, in discordance analyses, among participants with absolute difference of total triglycerides and mean triglyceride in LDL particles above the 10th percentile, total triglycerides were not associated with CHD after accounting for VLDL particle concentrations or HDL cholesterol (HDL‐C) and non–HDL‐C, whereas triglycerides in LDL remained as a significant marker of CHD risk (Table S7).
Table 4.
Lipoprotein Particle Composition Versus CHD Risk
| Lipoprotein type | Particle composition, molecules/particle | A: adjusted for age and sex only | B: adjusted for age, sex, and other confounders | C: further adjusted for lipoprotein particle concentration | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline mean | Usual SD | HR (99% CI) | LR χ2 | HR (99% CI) | LR χ2 | HR (99% CI) | LR χ2 | |
| VLDL | ||||||||
| Cholesterol | 5022.8 | 226.3 | 1.10 (1.05–1.15) | 29.0 | 1.10 (1.05–1.15) | 26.6 | 0.97 (0.90–1.03) | 1.9 |
| Cholesteryl esters | 3033.7 | 179.0 | 1.00 (0.96–1.04) | <0.1 | 1.04 (0.99–1.08) | 4.9 | 1.00 (0.94–1.07) | <0.1 |
| Free cholesterol | 1989.1 | 129.3 | 1.22 (1.17–1.28) | 118.1 | 1.15 (1.09–1.21) | 48.7 | 0.92 (0.86–0.99) | 8.6 |
| Phospholipids | 3189.5 | 270.8 | 1.23 (1.17–1.28) | 134.7 | 1.14 (1.08–1.19) | 45.6 | 0.94 (0.87–1.02) | 3.7 |
| Triglycerides | 5880.2 | 1394.6 | 1.10 (1.06–1.15) | 36.3 | 1.03 (0.99–1.08) | 3.0 | 0.93 (0.87–1.00) | 7.1 |
| IDL | ||||||||
| Cholesterol | 2716.4 | 185.8 | 0.79 (0.76–0.83) | 180.6 | 0.86 (0.82–0.90) | 65.0 | 0.99 (0.91–1.08) | 0.1 |
| Cholesteryl esters | 2001.6 | 140.6 | 0.79 (0.76–0.83) | 184.2 | 0.86 (0.82–0.90) | 69.7 | 0.98 (0.90–1.07) | 0.4 |
| Free cholesterol | 714.8 | 48.4 | 0.83 (0.80–0.87) | 116.5 | 0.90 (0.86–0.95) | 33.0 | 1.01 (0.95–1.08) | 0.1 |
| Phospholipids | 945.8 | 56.3 | 0.90 (0.86–0.94) | 40.4 | 0.96 (0.91–1.00) | 6.6 | 1.03 (0.98–1.09) | 2.8 |
| Triglycerides | 313.6 | 61.0 | 1.11 (1.07–1.15) | 47.4 | 1.06 (1.02–1.10) | 15.1 | 1.12 (1.05–1.19) | 19.3 |
| LDL | ||||||||
| Cholesterol | 1445.5 | 71.0 | 0.85 (0.82–0.89) | 91.6 | 0.90 (0.86–0.94) | 37.0 | 1.03 (0.94–1.14) | 0.8 |
| Cholesteryl esters | 1055.0 | 45.3 | 0.89 (0.85–0.92) | 52.4 | 0.92 (0.88–0.96) | 22.7 | 1.03 (0.95–1.12) | 0.8 |
| Free cholesterol | 390.5 | 32.4 | 0.83 (0.80–0.87) | 129.5 | 0.89 (0.85–0.93) | 48.9 | 1.02 (0.92–1.14) | 0.3 |
| Phospholipids | 506.2 | 22.2 | 0.85 (0.81–0.89) | 94.6 | 0.88 (0.84–0.92) | 56.5 | 1.06 (0.98–1.14) | 4.0 |
| Triglycerides | 118.1 | 20.4 | 1.11 (1.07–1.15) | 47.6 | 1.05 (1.01–1.09) | 11.4 | 1.18 (1.10–1.26) | 34.4 |
| HDL | ||||||||
| Cholesterol | 87.0 | 9.9 | 0.74 (0.70–0.78) | 240.5 | 0.81 (0.76–0.86) | 96.7 | 0.96 (0.89–1.03) | 2.2 |
| Cholesteryl esters | 67.6 | 7.8 | 0.73 (0.69–0.76) | 283.8 | 0.79 (0.75–0.83) | 125.4 | 0.93 (0.86–1.01) | 5.0 |
| Free cholesterol | 19.4 | 2.2 | 0.85 (0.81–0.90) | 66.9 | 0.93 (0.89–0.99) | 10.6 | 1.01 (0.95–1.07) | 0.1 |
| Phospholipids | 102.1 | 7.1 | 0.87 (0.83–0.91) | 63.3 | 0.91 (0.87–0.95) | 25.8 | 1.02 (0.96–1.09) | 0.9 |
| Triglycerides | 9.5 | 2.4 | 1.22 (1.18–1.27) | 174.9 | 1.15 (1.11–1.20) | 81.0 | 1.16 (1.03–1.31) | 10.4 |
HRs per usual SD higher level of average molecules of the lipid per lipoprotein particle among 89 422 participants. HRs calculated by Cox proportional hazards models with: (A) stratification by age and sex; (B) model A with further adjustment for ethnicity, education, region, Townsend Deprivation Index, smoking, alcohol, and body mass index; and (C) model B with further adjustment for concentrations of VLDL, LDL, and HDL particles (not further adjusted for IDL concentration because of its high correlation with LDL concentration). LR χ2 improvement with the addition of the exposure variable to the model with stated adjustments. “Usual SD” was obtained from the baseline SD through multiplication by square root of the regression dilution ratio, representing an unbiased estimate of the long‐term average level in each baseline‐defined group. CHD indicates coronary heart disease; HDL, high‐density lipoprotein; HR, hazard ratio; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; LR, likelihood ratio; and VLDL, very‐low‐density lipoprotein.
Figure 2. Total triglycerides and triglycerides in low‐density lipoprotein (LDL) vs coronary heart disease risk, adjusted for lipoprotein particle concentration.
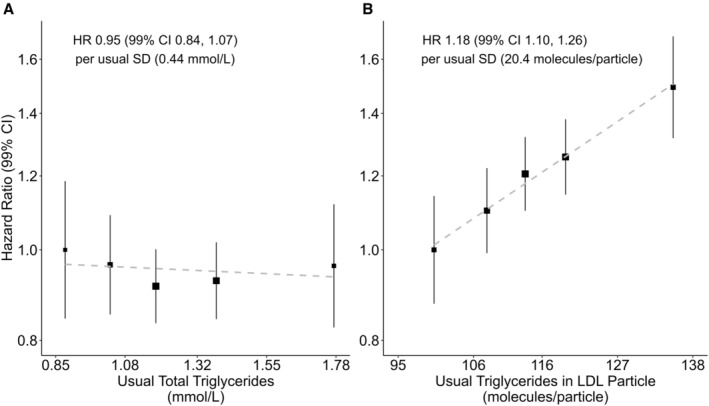
Hazard ratios (HRs) per usual SD higher level of triglycerides among 89 422 participants (stratified by quintiles). A, Total triglycerides. Total triglyceride is triglyceride concentration in all lipoprotein particles. B, Triglycerides in LDL. Triglyceride in LDL is average triglyceride molecules per LDL particle. HRs calculated by Cox proportional hazards models stratified by age and sex and adjusted for ethnicity, education, region, Townsend Deprivation Index, smoking, alcohol, body mass index, and concentration of very‐low‐density lipoprotein, LDL, and high‐density lipoprotein particles. “Usual” levels are estimated from the mean value at resurvey within each baseline‐defined group, providing an unbiased estimate of the long‐term average level in each baseline‐defined group. “Usual SD” was obtained from the baseline SD through multiplication by square root of the regression dilution ratio. Area of the square is inversely proportional to the variance of the category‐specific log risk. CI indicates confidence interval; HDL, high‐density lipoproteins; HR, hazard ratio; LDL, low‐density lipoproteins; SD, standard deviation; and VLDL, very‐low‐density lipoproteins.
Total ApoB concentration (also measured by Nightingale NMR) was highly correlated with LDL particle concentration (r=0.99; Table S8), and the strength of the association between total ApoB level and CHD risk was the same as LDL particle concentration (Figures S5). ApoB concentration was still highly but relatively more weakly correlated with VLDL (r=0.87). Given total ApoB concentration, concentrations of VLDL and HDL particles remained positively associated with CHD risk, whereas the association of total ApoB level given VLDL concentration attenuated to null (Figures S6 and S7).
In sensitivity analyses, further adjustment for other potential confounders did not materially change the main associations, and neither did mutual adjustment of other particle concentrations for the association of lipoprotein concentrations (Table S9). Furthermore, exclusion of the first 2 years of follow‐up to assess for any impact of reverse causality to the metabolite measures attributable to the subclinical disease at baseline (although without formal diagnosis) did not change the associations. Supplementary analyses showed high consistency among lipid and apolipoprotein measurements using traditional clinical chemistry and those using Nightingale NMR (although the absolute values were different because UK Biobank used serum sample for clinical chemistry measurements and plasma sample for NMR measurement) 17 (Table S10), and the analyses of associations to CHD risk were not statistically different between the 2 types of measurements (Table S11). Lipid content measures also showed similar results with our derived lipid composition measures (Table S6 versus Table 4) but wider CIs when assessing the associations after adjusting for particle concentrations attributable to the collinearity with lipid content measures.
Discussion
In this large‐scale prospective study, CHD risk was positively associated with VLDL, IDL, and LDL particle concentrations and inversely associated with HDL particle concentration. There was no evidence that the strength of these associations varied by sizes of LDL and HDL particles, but larger subclasses of VLDL were found to be less strongly related to CHD risk. After adjusting for lipoprotein particle concentrations, there was strong evidence of a positive association between mean triglyceride molecules per LDL particle and CHD risk but little evidence of associations with other lipid fractions or in VLDL or HDL particles. Of the lipoprotein characteristics assessed, CHD risk was most strongly related to lipoprotein particle concentration, and separate measurements of lipoprotein concentrations may be of greater value than the measurement by ApoB, which was largely determined by LDL concentration alone.
Several previous studies have also assessed the associations of NMR‐derived measures of lipoprotein concentrations with CHD risk. 18 , 19 , 20 These studies have consistently described positive associations with VLDL and LDL particle concentrations and inverse associations with HDL particle concentrations, as in the present report. However, the associations in these studies are somewhat weaker than in the present report, likely reflecting associations with baseline measures rather than long‐term average (“usual”) levels of lipoprotein concentration. For example, the placebo arm of a large randomized trial of simvastatin versus placebo showed adjusted HRs per 1‐SD higher level were 1.11 (95% CI, 1.05–1.17; SD=0.34 nmoL/mL) for LDL particles concentration and 0.88 (95% CI, 0.83–0.92; SD=5.18 nmoL/mL) for HDL particle concentration. 18
Subclasses of lipoproteins can be categorized by either particle size or density. Previous studies on the association of size‐determined lipoprotein subclasses with CHD risk focused mainly on LDL and HDL. 8 , 18 , 19 Consistent with the present report, these studies did not find differences in the strength of associations across LDL subclasses. 8 However, the evidence for the associations of CHD risk with HDL subclasses has been less consistent, with some describing heterogeneity and others not. 8 , 18 , 19 The reason for this is unclear but may reflect different sets of adjustments; studies that have further adjusted for LDL concentration tend to find little evidence of heterogeneity among HDL subclasses. 18 , 19 Similarly, although HDL size was significantly associated with CHD risk in some studies, 8 there was little evidence of associations of LDL and HDL particle size after accounting for particle concentrations. 18 , 21 One possible explanation is that although smaller particles are easier to penetrate into the artery wall, the ratio of surface cholesterol/phospholipid in the particle also decreases significantly as the size becomes smaller, leading to a decrease in binding affinity and therefore making it equally possibile to be trapped within the arterial intima. 22 , 23 However, some small variations in atherogenicity observed among lipoproteins in different size are more likely attributable to the variations in concentrations. On the other hand, small dense LDL, another subclass of LDL categorized by density, has been found to be more atherogenic than other lipoproteins. 22 , 24 This is probably because small dense LDL is mostly derived from triglyceride‐rich LDL, following a different hydrolysis mechanism in hypertriglyceridemic status, and its more atherogenic property is probably attributable to being susceptible to chemical modification, which increases its atherogenicity. 22 , 25
We also examined the effect on CHD risk with variation in lipid composition of lipoproteins, adjusting for lipoprotein concentration. Standard measures of plasma lipids are the sum of the lipid carried by lipoprotein particles and therefore strongly correlated with particle concentration, which limits the potential to assess their separate effects. In contrast to previous studies, we divided the lipid concentration by the corresponding lipoprotein particle concentration to examine the associations with average lipid composition (molecules per particle) for each type of lipoprotein, which is not as strongly correlated with lipoprotein concentration. Associations with lipid composition showed substantial attenuation on adjustment for potential confounders and particle concentrations, with the exception of mean triglyceride molecules per LDL particle. Although large observational studies have demonstrated a strong positive association of LDL cholesterol and remnant lipoprotein particle (composed primarily of VLDL and IDL) cholesterol with cardiovascular disease, 26 , 27 , 28 in the present report there was little association of mean molecules of cholesterol (either esterified cholesterol or free cholesterol or adding both) in any type of lipoprotein after adjusting for particle concentrations.
The consistency between biomarkers measured by Nightingale NMR and by clinical chemistry has been shown in 3 large cohorts, including UK Biobank. 8 , 9 Before the application of Nightingale NMR, several previous studies have indirectly assessed the effect of lipoprotein particle composition on CHD risk by comparing the strengths of associations of ApoB‐containing particles concentration and those with standard measures of plasma lipids. 1 , 15 , 16 , 29 , 30 These studies have found similar associations of total ApoB concentration, LDL cholesterol, and non–HDL‐C to CHD risk. However, few have directly assessed the relevance of particle composition adjusting for particle concentration. A recent cohort study also found that the association with myocardial infarction was best captured by the number of ApoB‐containing lipoproteins, rather than standard measures of lipid content. 4 Furthermore, 2 Mendelian randomization studies have supported the causality of the relation between ApoB and CHD risk but not with plasma cholesterol after accounting for total ApoB concentration. 31 , 32 It has been suggested that cholesterol enters the arterial wall within ApoB‐containing particles and, as such, the number of ApoB‐containing particles within the lumen of an artery may be the primary determinant of atherosclerosis. 33
In our study, total ApoB concentration was strongly correlated with LDL concentration but less strongly correlated with VLDL. Total ApoB concentration may only capture the variation in LDL concentration but fail to adequately capture the variations in other ApoB‐containing lipoproteins with much lower concentrations. The strong association of VLDL concentration given ApoB indicates that VLDL concentration is relevant to CHD risk over and above that of total ApoB concentration alone and highlights the importance of using direct measures of lipoprotein concentration to assess the separate effects of other lipoprotein characteristics.
Accumulating epidemiologic and genetic evidence has supported increased triglycerides as an additional cause of cardiovascular disease, 2 , 3 , 34 whereas uncertainty still existed on whether triglycerides provide additional information on vascular risk after accounting for cholesterol or ApoB levels. 1 , 26 Our results showed little evidence for an association with mean triglyceride molecule levels in VLDL particle but positive associations with triglycerides in LDL. This finding is consistent with a recent genetic study showing that triglycerides in LDL are associated with increased CHD risk in models adjusted for cholesterol levels. 35 One hypothesis is that excess triglycerides in LDL, generated in hypertriglyceridemic states, will finally convert to small, dense LDL, which was found to be closely associated with increased CVD risk. 24 , 25 , 35 Given lipoprotein concentrations, our results found that the addition of single measurement of LDL triglyceride to the model improved the likelihood ratio χ2 statistic, whereas little evidence of improvement was shown with the addition of total triglycerides. Discordance analysis also showed that after accounting for HDL‐C and non–HDL‐C (as measured by Nightingale NMR) or lipoprotein concentrations, LDL triglyceride remained significantly associated with CHD risk, whereas the association of total triglycerides was largely accounted for by the concentration of VLDL particles. These differences may partly explain the previous inconsistency in the evidence on triglycerides, which mostly used total triglyceride concentration as the exposure. Therefore, our findings indicated the value of disaggregating the standard measures of total triglycerides by type of lipoprotein particle. Further work is necessary to assess causality, but if causal, triglycerides in LDL might become a valuable additional biomarker of CHD risk.
This study has several key strengths. This is one of few large‐scale studies to robustly assess the separate associations of lipoprotein particle concentration, size, and composition. Although multiple metabolite measurements allowing for assessment of within‐person variation were not available for all participants, the study conducted a resurvey to allow correction for regression dilution, enabling estimates of associations with long‐term average levels of lipoprotein characteristics. Furthermore, linkage to National Health Service electronic health records and national death registries limited loss to follow‐up and allowed reliable ascertainment of CHD events. Although these analyses assessed the relevance of lipoprotein characteristics after adjustment for each other, it is not possible to exclude the potential for residual confounding (because of confounders not measured or measured with random noise) or reverse causality in observational studies. Lack of measurement of the sizes of lipoprotein subclasses and the lipid density of lipoprotein limited a deeper exploration of relevant mechanisms and predictive values, which needs to be considered when additional data on metabolomics are released by UK Biobank. It was not possible in this study to directly compare results for previous NMR methods and other measurement techniques with the current Nightingale NMR method, so the comparison with older studies is based on the assumption that the 2 methods would give comparable results. Because of some concerns related to the validity of Nightingale NMR measures, 36 more evidence of comparing Nightingale NMR with other techniques is needed. Finally, although UK Biobank is not representative of the UK population, it has been shown that associations with health outcomes can be generalized. 37 Future analyses should further assess the effect modification by other metabolic factors and the causality of these associations, including using Mendelian randomization approaches.
Conclusions
This large‐scale prospective study uses Nightingale NMR to quantify the associations of lipoprotein particle concentration, size, and lipid composition with CHD risk. CHD risk was most strongly related to VLDL, LDL, and HDL particle concentrations, but there was some additional effect from mean triglyceride molecules per LDL particle. The results also suggest that separate measurement of VLDL and LDL particles may be superior to measurement of total ApoB levels, which was found to be highly correlated with LDL particle concentration alone.
Sources of Funding
The Clinical Trial Service Unit and Epidemiological Studies Unit receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry; further details can be found at https://www.ndph.ox.ac.uk/files/about/ndph‐independence‐of‐research‐policy‐jun‐20.pdf. This research used UK Biobank data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (grant reference MC_PC_20058).
Disclosures
Dr Lewington reports grants from the Medical Research Council and research funding from the US Centers for Disease Control and Prevention Foundation (with support from Amgen) and from the World Health Organization during the conduct of the study. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S11
Figures S1–S7
Acknowledgments
This research used the UK Biobank resource (application number 30418). We appreciate the participants of UK Biobank for their contribution to the resource. This work uses data provided by patients and collected by the National Health Service as part of their care and support. Nightingale Health Plc approved the use of data from application 30418.
This article was sent to Amgad Mentias, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029552
For Sources of Funding and Disclosures, see page 10.
References
- 1. Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver‐Williams C, Wood AM, Butterworth AS, et al. Association of triglyceride‐lowering LPL variants and LDL‐C‐lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W, Watkins H, Samani NJ, Saleheen D, et al. Triglyceride‐mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marston NA, Giugliano RP, Melloni GEM, Park JG, Morrill V, Blazing MA, Ference B, Stein E, Stroes ES, Braunwald E, et al. Association of apolipoprotein B‐containing lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis: distinguishing between particle concentration, type, and content. JAMA Cardiol. 2022;7:250–256. doi: 10.1001/jamacardio.2021.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soininen P, Kangas AJ, Würtz P, Suna T, Ala‐Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8:192–206. doi: 10.1161/CIRCGENETICS.114.000216 [DOI] [PubMed] [Google Scholar]
- 6. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 7. Needham LL, Smy L, Lee MA, Kunzler TM, Genzen JR. Phlebotomy tube interference with nuclear magnetic resonance (NMR) lipoprotein subclass analysis. Clin Chim Acta. 2019;488:235–241. doi: 10.1016/j.cca.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 8. Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, Guo Y, Xu X, Bian Z, Hu R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–632. doi: 10.1016/j.jacc.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Julkunen H, Cichońska A, Tiainen M, Koskela H, Nybo K, Mäkelä V, Nokso‐Koivisto J, Kristiansson K, Perola M, Salomaa V, et al. Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK biobank. Nat Commun. 2023;14:604. doi: 10.1038/s41467-023-36231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terminology and Classifications Delivery Service . National Clinical Coding Standards OPCS‐4. 2021. Accessed May 31, 2021. https://classbrowser.nhs.uk/ref_books/OPCS‐4.9_NCCS‐2021.pdf.
- 12. Clarke R, Emberson JR, Breeze E, Casas JP, Parish S, Hingorani AD, Fletcher A, Collins R, Smeeth L. Biomarkers of inflammation predict both vascular and non‐vascular mortality in older men. Eur Heart J. 2008;29:800–809. doi: 10.1093/eurheartj/ehn049 [DOI] [PubMed] [Google Scholar]
- 13. Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23:93–104. doi: 10.1002/sim.1485 [DOI] [PubMed] [Google Scholar]
- 14. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet (London, England). 2002;360:1903–1913. doi: 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 15. Sniderman AD, Islam S, Yusuf S, McQueen MJ. Discordance analysis of apolipoprotein B and non‐high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis. 2012;225:444–449. doi: 10.1016/j.atherosclerosis.2012.08.039 [DOI] [PubMed] [Google Scholar]
- 16. Welsh C, Celis‐Morales CA, Brown R, Mackay DF, Lewsey J, Mark PB, Gray SR, Ferguson LD, Anderson JJ, Lyall DM, et al. Comparison of conventional lipoprotein tests and apolipoproteins in the prediction of cardiovascular disease. Circulation. 2019;140:542–552. doi: 10.1161/CIRCULATIONAHA.119.041149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The UK Biobank . Nightingale Health Metabolic Biomarkers: Phase 1 Release. 2020. Accessed May 31, 2022. https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/nmrm_companion_doc.pdf.
- 18. Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R; Heart Protection Study Collaborative Group . Lipids and lipoproteins and risk of different vascular events in the MRC/BHF heart protection study. Circulation. 2012;125:2469–2478. doi: 10.1161/CIRCULATIONAHA.111.073684 [DOI] [PubMed] [Google Scholar]
- 19. Akinkuolie AO, Paynter NP, Padmanabhan L, Mora S. High‐density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes. 2014;7:55–63. doi: 10.1161/CIRCOUTCOMES.113.000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El Harchaoui K, Arsenault BJ, Franssen R, Després JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, et al. High‐density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006 [DOI] [PubMed] [Google Scholar]
- 22. Ikezaki H, Lim E, Cupples LA, Liu CT, Asztalos BF, Schaefer EJ. Small dense low‐density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham offspring study. J Am Heart Assoc. 2021;10:e019140. doi: 10.1161/JAHA.120.019140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNamara JR, Small DM, Li Z, Schaefer EJ. Differences in LDL subspecies involve alterations in lipid composition and conformational changes in apolipoprotein B. J Lipid Res. 1996;37:1924–1935. doi: 10.1016/S0022-2275(20)37557-X [DOI] [PubMed] [Google Scholar]
- 24. Hirayama S, Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin Chim Acta. 2012;414:215–224. doi: 10.1016/j.cca.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 25. Miller M. Low‐density lipoprotein triglycerides: widening the atherogenic landscape in CVD risk assessment. J Am Coll Cardiol. 2018;72:170–172. doi: 10.1016/j.jacc.2018.03.541 [DOI] [PubMed] [Google Scholar]
- 26. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varbo A, Benn M, Tybjærg‐Hansen A, Jørgensen AB, Frikke‐Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026 [DOI] [PubMed] [Google Scholar]
- 28. Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS, Blaha MJ, Kulkarni KR, Correa A, D'Agostino RB Sr, et al. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson heart and Framingham offspring cohort studies. J Am Heart Assoc. 2016;5:5. doi: 10.1161/JAHA.115.002765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mora S, Buring JE, Ridker PM. Discordance of low‐density lipoprotein (LDL) cholesterol with alternative LDL‐related measures and future coronary events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case‐control study. Lancet (London, England). 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4 [DOI] [PubMed] [Google Scholar]
- 31. Richardson TG, Sanderson E, Palmer TM, Ala‐Korpela M, Ference BA, Davey Smith G, Holmes MV. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med. 2020;17:e1003062. doi: 10.1371/journal.pmed.1003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, Packard CJ, Laufs U, Brook RD, Oliver‐Williams C, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318:947–956. doi: 10.1001/jama.2017.11467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019;4:1287–1295. doi: 10.1001/jamacardio.2019.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet (London, England). 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 35. Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, Guild CS, Boerwinkle E, Ballantyne CM, et al. Remnant‐like particle cholesterol, low‐density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol. 2018;72:156–169. doi: 10.1016/j.jacc.2018.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krauss RM, Remaley AT, John Chapman M. Concerns regarding NMR lipoprotein analyses performed on the nightingale heath platform–focus on LDL subclasses. J Clin Lipidol. 2022;16:250–252. doi: 10.1016/j.jacl.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 37. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health‐related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S11
Figures S1–S7


