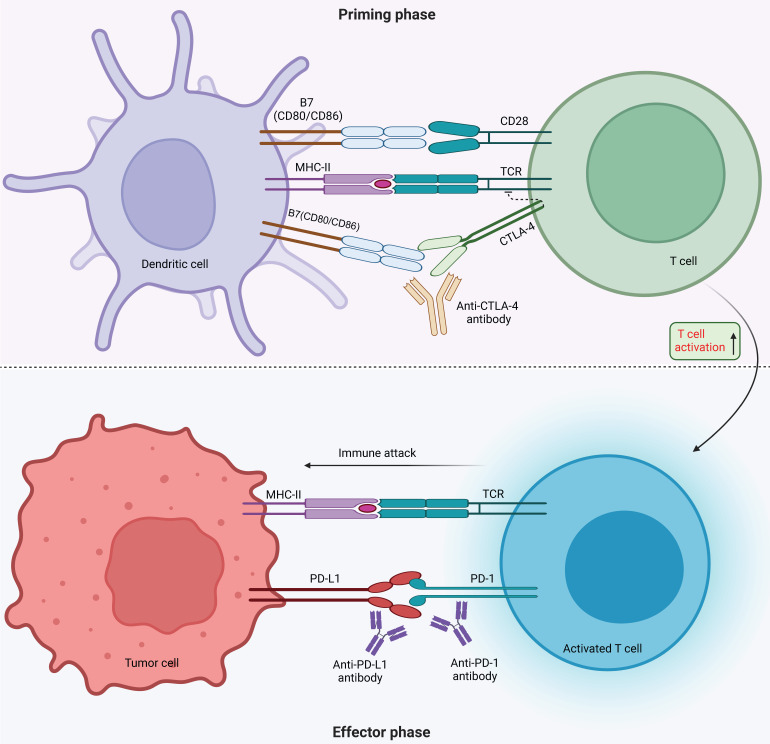
Figure 1.
Mechanism of immune checkpoint inhibitors. Monoclonal antibodies targeting CTLA-4 and PD-1 receptors, as well as PD-L1, are ICIs that regulate T cell activation. During the priming phase, antigen presentation by MHC class II molecules on antigen-presenting cells triggers T cell activation by the T-cell receptor (TCR) recognizing antigen, followed by CD28 receptor binding with B7 (CD80 or CD86). The surface receptor of CTLA-4 on T cells inhibits T cell activation through competing with CD28 for CD80 or CD86 binding. The use of CTLA-4 inhibitor antibodies blocks the CTLA-4-CD80 or CTLA-4-CD86 binding, thus promoting T cell activation (indicated by a dashed line). During the effector phase, PD-1 expressed by T cells interacts with PD-L1 expressed by tumor and myeloid cells, promoting apoptosis of antigen-specific T cells while reducing regulatory T cell apoptosis. Under normal circumstances, this mechanism serves to protect against autoimmune disorders. However, cancer cells take advantage of it by increasing the expression of PD-L1, which helps them evade the immune system. To counteract this, inhibitors of PD-1 and PD-L1 can be used to block their interaction and promote activation of T-cells.