Abstract
Diabetes is a major global public health problem with high incidence and case fatality rates. Traditional Chinese medicine (TCM) is used to help manage Type 2 Diabetes Mellitus (T2DM) and has steadily gained international acceptance. Despite being generally accepted in daily practice, the TCM methods and hypotheses for understanding diseases lack applicability in the current scientific characterization systems. To date, there is no systematic evaluation system for TCM in preventing and treating T2DM. Metabonomics is a powerful tool to predict the level of metabolites in vivo, reveal the potential mechanism, and diagnose the physiological state of patients in time to guide the follow-up intervention of T2DM. Notably, metabolomics is also effective in promoting TCM modernization and advancement in personalized medicine. This review provides updated knowledge on applying metabolomics to TCM syndrome differentiation, diagnosis, biomarker discovery, and treatment of T2DM by TCM. Its application in diabetic complications is discussed. The combination of multi-omics and microbiome to fully elucidate the use of TCM to treat T2DM is further envisioned.
Keywords: metabolomics, traditional Chinese medicine, type 2 diabetes mellitus, diabetic complications, diagnosis
Introduction
Diabetes, a complex metabolic disorder, involves imbalances in blood sugar, insulin resistance, and dysfunction of islet β-cells. Over 90% of diabetes cases are type 2 diabetes (T2DM).1,2 T2DM stems from disruptions in pancreatic hormone secretion, insulin action on target tissues, and defects in insulin signaling mechanisms, impacting carbohydrate and lipid metabolism.3 This condition significantly impairs patients’ quality of life, and its prevalence and treatment costs pose substantial social and economic burdens. Advanced T2DM can lead to severe complications and death, yet suitable prevention and treatment approaches remain elusive.4
In traditional Chinese medicine (TCM), diabetes is considered as Xiaoke, which means “consumptive thirst”. It can be differentially diagnosed based on its various symptoms and patterns. One common type is the yin deficiency and fire pattern, which is characterized by polydipsia, polyuria, red cheeks, irritability, a red tongue with yellow fur, and a rapid pulse.5 TCM has a long history of treating diabetes and its complications. The multiple component and target characteristics of TCM have unique advantages in the prevention and control of complex diseases such as diabetes.6,7 However, due to the lack of scientific and modern evidence, TCM is also facing serious challenges in the diagnosis and treatment of diabetes. New technologies must be developed quickly to improve the detection of T2DM risk factors and the T2DM prevention, diagnosis, and prognosis of TCM, potentially impacting the early diagnosis and personalized care of patients with T2DM.
Metabolomics can systematically evaluate the changes of metabolites in the body and screen metabolic biomarkers, thereby enhancing the effectiveness of disease diagnosis and treatment.8 Its approach and properties are similar to TCM’s holistic concept, which offers opportunities and challenges for TCM modernization.9–11 Metabolomics can benefit from the systematic analyses of metabolites and the discovery of various biomarkers and interfering pathways in TCM syndromes or after TCM treatment to clarify the mechanism of TCM and the significance of evidence-based TCM.12 Metabolomics has the potential as a holistic approach for clinical diagnosis and care and as a way to better elucidate the pathological mechanism of T2DM.
In this context, our discussion revolves around the application of metabolomics to elucidate TCM’s principles in T2DM syndrome differentiation, diagnosis, and anti-diabetic mechanisms. We explore its potential in treating diabetic complications and propose the integration of multi-omics and microbiomes to comprehensively understand the mechanisms underlying TCM’s preventive and therapeutic approaches for T2DM. This not only provides a theoretical basis but also introduces a novel perspective for the modernization of TCM.
Metabolomics in TCM Syndrome Differentiation of T2DM
In TCM, diabetes, known as xiaokezheng, is characterized by symptomatic polydipsia with an extensive treatment history.13 TCM emphasizes syndrome differentiation as the foundation for treating diseases, including diabetes.14 According to TCM theory, diseases result from imbalances in Yin, Yang, Qi, blood, organs, and meridians,15 with diabetes pathogenesis involving Yin deficiency and excessive heat syndrome in the early phase, deficiency of Qi and Yin syndrome in the middle stage, and deficiency of Yin and Yang syndrome in the later phase.16 Inconsistent T2DM diagnoses among TCM physicians highlight the need for objective indicators to enhance syndrome accuracy.17,18
TCM syndromes and metabolic characteristics are interconnected, providing mutual validation and aiding modern biology in confirming TCM syndromes for diabetes diagnosis and treatment. Metabolomics, a systematic study of metabolites after environmental stimulation, allows a holistic investigation of changes in human metabolism concerning diseases.19 This holistic view aligns with TCM concepts and scientifically conveys the sense of TCM syndromes.20 Analysis of endogenous plasma metabolites in patients with Kidney-Yin Deficiency Syndrome (KYDS) and diabetes revealed specific changes, supporting the syndrome differentiation principle in TCM.21
While metabonomics alone has limitations in clarifying the biological essence of syndromes, future research should target upstream functional proteins and genes based on metabolic markers. This approach promises a more in-depth and specific analysis of syndromes, leading to more accurate treatments due to the complexity of syndromes.
Application of Metabolomics in the Diagnosis of T2DM
Pre-diabetes is an intermediate stage between normal glucose tolerance (NGT) and T2DM,22 which is often difficult to diagnose accurately. T2DM increases the risk of cardiovascular disease (CVD) and microvascular complications by 2- to 4-fold, which may have existed before diagnosis.23 Since early intervention may be the most effective way to delay the progress of T2DM, it is crucial to develop sensitive biomarkers for early diagnosis of various pathologies related to T2DM. Metabolomics is a powerful method to find biomarkers. It can acquire a new view of disease pathophysiology and identify individual metabolites or their profiles as potential biomarkers to distinguish normal and pathological states.24 Accurate biomarkers are necessary for better diagnosis and prognosis, guiding molecular targeted therapy and exploring treatment response and results.25 One of the significant breakthroughs in enhancing the diagnosis of risk factors for T2DM was the discovery and validation of biomarkers. The identified potential biomarkers in metabolomics-based T2DM in the last five years have been summarized in Table 1. These findings help elucidate the pathogenic mechanisms underlying T2DM and may aid in diagnosing and treating T2DM patients.
Table 1.
Biomarker Discovery in T2DM
| Prediction | Detective Method | Metabolic biomarkers | Ref. |
|---|---|---|---|
| Pre-diabetes | LC-MS | Valine, isoleucine, tryptophan, lysine, arginine, proline | [26] |
| Pre-diabetes | LC-MS | Hexose, 2-hydroxybutyric/2-hydroxyisobutyric acid, and phenylalanine | [27] |
| Pre-diabetes | 1D 1H NMR, FIA-MS, LC-MS | BCAAs, trimethyl uric acid, trimethylamine N-oxide, lysophosphatidylcholines, β-hydroxybutyrate, phosphatidylcholines, Alanine, acylcarnitine, sphingomyelins, amino acids; Glutamine and SM.C16.1, Lyso.PC.a.C18.0, PC.ae.C34.2, C3. DC. C4.OH, | [28] |
| Pre-diabetes | GC–MS | Tyrosine, lysine, alanine, valine, proline, phenylalanine, tryptophan, hexadecanoic acid, alpha-ketoglutaric acid, myristic acid, octadecanoic acid, uric acid | [29] |
| Pre-diabetes and T2DM | LC-MS | Ceramides, saturated sphingomyelins, unsaturated sphingomyelins, hydroxyl-sphingomyelins, and a hexosylceramide | [30] |
| Pre-diabetes | LC-MS | N2, N2-dimethylguanosine, 7-methylguanine and 3-hydroxytrimethyllysine | [31] |
| Pre-diabetes | LC-MS | A cluster of saturated sphingomyelins | [32] |
| Pre-diabetes | 1H-NMR | Lipids in HDL subtypes, citrate, glycoprotein acetyls, branched-chain amino acids, VLDL lipids | [33] |
| Pre-diabetes | LC-MS | Alanine, aspartate, glutamate, isoleucine, leucine, phenylalanine, tyrosine, tryptophan, and valine | [34] |
| Pre-diabetes | GC-MS and LC-MS | 1,5-anhydroglucitol | [35] |
| Pre-diabetes | HPLC-MRM | TAGs, lyso-phosphatidylinositols, phosphatidylcholines, PUFA-PEps, cholesteryl esters | [36] |
| Pre-diabetes | CIL-LC-MS | Methionine (Met) sulfoxide, amino acids (Asn, Gln, and His), 2-methyl-3-hydroxy-5-formylpyridine-4-carboxylate, L-2-amino-3-oxobutanoic acid, serotonin, and 4,6-dihydroxyquinoline. | [37] |
| Pre-diabetes | GC-MS and LC-MS | Lysophosphatidylcholines (muscle), glycodeoxycholic acid (liver) | [38] |
| Pre-diabetes and diabetes | GC-MS | Maltose, glucose, trehalose, an unknown sugar compound (U15), mannose, fructose, sedoheptulose, and 1,5 anhydroglucitol, | [39] |
| T2DM | CIL-LC-MS | Amino acids, amino acids metabolites, and dipeptides. | [37] |
| T2DM | GC-MS and LC-MS | Carnitines (liver), lysophosphatidylcholines (muscle and serum) | [38] |
| T2DM | LC-MS | Alanine, 3-methyl histidine, glutamic acid, arginine, tryptophan, and ethanolamine sarcosine | [40] |
| T2DM | 1H-NMR and 2D-NMR | 61 distinct metabolites | [41] |
| Pre-diabetes and diabetes | LC-MS | Oxidized glycerophosphatidylcholines | [42] |
| Obese insulin sensitive (OIS) | LC-MS | Phospholipid metabolites, including choline, glycerophosphoethanolamine, and glycerophosphorylcholine | [43] |
| T2DM | GC-MS | Tyrosine, alanine, valine, tryptophan, and alpha-ketoglutaric acid | [29] |
| T2DM | LC-MS | Organophosphate flame retardant (OPFR) diesters | [44] |
| T2DM | LC-MS | Dimethylguanidino valerate, acisoga, acylcarnitine C10:3, homocitrulline, N2, 1-methyladenosine, N2-dimethylguanosine, hippurate, urobilin, threonine, lysine and tryptophan | [45] |
| T2DM | LC-MS | Urine 3-hydroxyundecanoyl-carnitine | [46] |
| Pre-diabetes and T2DM | NMR | Branched-chain, aromatic amino acids, linoleic n-6 fatty acid, triacylglycerol within VLDL particles, and non-esterified cholesterol in large HDL particles | [47] |
| T2DM | GC-MS | Lysophosphatidylcholine, deoxycholic- and glycodeoxycholic acid, all BCAAs, and their catabolic intermediates | [48] |
| Pre-diabetes | GC-MS | Lysophosphatidylcholine, ursodeoxycholic- and chenodeoxycholic acid, leucine and its catabolic intermediates (ketoleucine and C5-carnitine) | [48] |
Metabolomic Profiles for the Prediction of T2DM
In the early stages, numerous studies have indicated elevated levels of branched-chain amino acids (BCAAs), their derivatives, aromatic amino acids, and α-hydroxybutyrate in plasma before T2DM, while glycine and glutamine show negative associations.49–51 Additionally, noticeable changes in serum concentrations of glycerophospholipids and sugar metabolites, including deoxyhexose sugars and sugar alcohols, occurred six years before the onset of T2DM.52 Notably, glyoxylate levels in human serum significantly increased up to three years before a T2DM diagnosis, suggesting its potential role in guiding novel antidiabetic therapies.53
Recent advancements in metabolomics have significantly contributed to pre-diabetes biomarker recognition.26,27,29–34,38 Li et al utilized non-targeted and targeted metabolomics to identify novel plasma biomarkers in lean β-Phb2-/- mice and obese db/db mice. The study highlighted 1,5-anhydroglucitol’s association with the loss of functional β-cell mass, revealing metabolic similarities between the liver and plasma.35
Lipidomics, a vital branch of metabolomics, demonstrated its significance in assessing lipid co-regulation changes before T2DM treatment. Lu et al employed a high-coverage targeted HPLC-MRM lipidomics method, revealing 38 lipids significantly related to T2DM, outperforming traditional clinical indices in predicting incident T2DM.36
Obesity, a key factor in metabolic diseases, including T2DM, is often associated with insulin resistance (IR). IR occurs several years before the clinical symptoms of T2DM, but due to hyperglycemia, IR prediction, diagnosis, and treatment have been delayed.54 Gu et al identified IR candidate biomarkers in serum, including methionine sulfoxide, L-2-amino-3-oxobutanoic acid, amino acids (Asn, Gln, and His), serotonin, and 2-methyl-3-hydroxy-5-formylpyridine-4-carboxylate, emphasizing the importance of early detection and intervention.37
Yun et al utilized high-coverage targeted lipidomics to identify sphingolipids associated with incident T2DM, providing insights into potential biochemical processes during pre-diabetes progression.30 These metabolomics findings offer guidance for early intervention strategies to delay T2DM onset.
Metabolomic Profiles for the Diagnosis of T2DM
Commonly used screening methods for T2DM, such as fasting plasma glucose (FPG) or glycated hemoglobin (HbA1c) analysis, often miss many affected individuals.27 Developing specific T2DM biomarkers is critical. High-throughput metabolomics studies systematically reviewed and meta-analyzed the relationship between metabolites and T2DM. Some metabolites, including alanine, leucine, glutamate, and others, were found to increase the risk of T2DM, while others like glutamine, serine, and lysophosphatidylcholine C18:2 were associated with reduced risk.55
Recent investigations have extensively studied diagnostic biomarkers associated with T2DM.41–45 Gu et al identified significant associations between 42 metabolites, including dipeptides and amino acids, in T2DM.37 Diamanti et al found higher carnitine levels in the liver of T2DM patients and lower lysophosphatidylcholines in muscle and serum.38 Mack et al discovered potential biomarkers, such as trehalose and various sugars, using semi-targeted GC-MS.39 A targeted metabolomics approach in functionally impaired older persons revealed specific amino acid and derivative profiles in those with T2DM.40
Furthermore, Zeng et al linked lysophosphatidylcholine, bile acids, and branched-chain amino acids (BCAAs) to T2DM in black South African women.48 Large-scale studies on urinary metabolomics identified 3-hydroxyundecanoyl-carnitine as a potential biomarker.46 Another analysis of four Finnish cohorts revealed potent T2DM risk biomarkers, including branched and aromatic amino acids and specific lipids.47
In summary, these studies highlight the association between metabolic alterations and T2DM. The metabolomics platform emerges as a powerful tool for T2DM screening.
Metabonomics Reveals the Efficacy and Mechanism of TCM in Treating T2DM
T2DM is a global health challenge commonly managed with oral hypoglycemic drugs and insulin injections.56 Chinese medicine, with its unique theories and extensive clinical applications, is gaining popularity globally.57 Metabolomic studies play a crucial role in understanding disease mechanisms, diagnostic markers, and the action of TCM, offering valuable insights for TCM research.58–60 Table 2 summarizes several groups’ experimental designs and metabolomics results.
Table 2.
Treatments for T2DM Evaluated by Metabolomics Approaches
| TCM | Sample Sources | Biological Samples | Detection Method | Metabolic Biomarkers | Metabolic Pathways | Ref. |
|---|---|---|---|---|---|---|
| Cicer arietinium L. | T2DM rats | Urine | LC-MS | Acylcarnitines, amino acid-related metabolites, and organic acids | Dicarboxylate and glyoxylate metabolism, vitamin B6 metabolism, tricarboxylic acid cycle, and energy metabolism | [61] |
| Naoxintong Capsule (NXT) | T2DM rats | Serum | LC-MS | L-carnitine, tyrosine, Tryptophan, Indoleacrylic acid, 11-Dehydrothromboxane B2, 3-Hydroxysebacic acid, etc. | Tyrosine, Phenylalanine, and tryptophan biosynthesis, glycerophospholipid metabolism, arachidonic acid metabolism, tyrosine metabolism, tryptophan metabolism, sphingolipid metabolism, and primary bile acid biosynthesis | [62] |
| Huanglian Decoction (HLD) | T2DM rats | Urine | LC-MS | Cytosine, L-carnitine, betaine, phenylalanine, glucose, citrate, phenylpyruvate, and hippuric acid | Glyoxylate and dicarboxylate metabolism, phenylalanine metabolism, and tricarboxylic acid (TCA) cycle | [63] |
| Astragalus Radix (HQ) and Dioscoreae Rhizoma (SY) | T2DM rats | Serum | 1H-NMR | Monoamine oxidases B, acetyl-CoA carboxylase 1, carbonic anhydrase 2, and catalase | Aminoacyl-tRNA biosynthesis, Valine, leucine, and isoleucine biosynthesis, Nitrogen metabolism, etc. | [64] |
| Melastoma dodecandrum Lour. (Melastomataceae) | T2DM rats | Serum | LC-MS | Cholic acid, taurine, nicotinuric acid, hippuric acid, phosphohydroxypyruvic acid, tyrosine, arachidonic acid, PGE2, phenylalanine, glucuronide, carnitine, phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositol | Lipid, amino acid, arachidonic acid, taurine, and nicotinic acid metabolism | [65] |
| Gardenia jasminoides fruits | T2DM rats | Urine | LC-MS | Kynurenic acid, 3-Oxo-4,6-choladienoic acid, Xanthurenic acid, Creatinine, Phenylacetylglycine, etc. | Bile acid biosynthesis, amino acid metabolism, vitamin B metabolism, taurine metabolism, etc. | [66] |
| Ginseng berry | T2DM rats | Serum | LC-MS | Shikimic acid, 5’ -Methylthioadenosine, 2-Isopropyl-3-oxosuccinate, Liothyronine, Glycocholic Acid, etc. | Bile acid metabolism, arachidonic acid metabolism, glucuronization | [67] |
| Lycii Cortex (LyC) | (db/db) mouse db/db mice | Serum | LC-MS | Circulating triglycerides, cholesterol, phosphatidylethanolamine, phosphatidylcholines, acylcarnitines | Regulating nuclear transcription factors | [68] |
| Mulberry (Morus multicaulis) branch bark powder | T2DM mice | Serum | GC-MS | Sugars, fatty, sugar alcohols, amino acids, and organic. | Lipid metabolism, carbohydrate metabolism, energy metabolism, protein metabolism, oxidative stress | [69] |
| BuZangTongLuo Formula (BZTLF) | T2DM mice | Serum | LC-MS | Geranylfarnesyl diphosphate (GFPP), enterobactin, lasaloid, deferoxamine, vanillic acid, 11-Hydroxyeicosatetraenoate glyceryl ester, cucurbitacin C, and angiotensin IV, koenigicine, 18-Carboxydinor-LTE4 and Ursodeoxycholic acid 3-sulfate | Glutathione metabolism, phosphatidylcholine biosynthesis, and tryptophan metabolism | [70] |
| The hypoglycemic decoction (HD) | T2DM rats | Urine | LC-MS | L-carnitine, 1-methyladenosine, 1-methylhistamine, 3-indoleacrylic acid, riboflavin, phenylalanine, atrolactic acid, 2-oxoglutarate, citrate, isocitrate, cortisol, and glucose | Glyoxylate metabolism, Tricarboxylic acid cycle, phenylalanine metabolism, and dicarboxylate metabolism | [71] |
| Huang-Lian-Jie-Du Decoction (HLJDD) | db/db mice | Plasma | LC-MS | D-Galactose, Vigabatrin, D-Glucose, Indoxyl sulfate, LysoPC, Eicosapentaenoic acid, Retinyl acetate, 2-oleoylglycerol, Docosapentaenoic acid | Fatty acid β-oxidation, glycerophospholipid metabolism, glucose metabolism, linoleic acid metabolism, and glutathione metabolism | [72] |
| Xiaokeyinshui extract combination (XEC) | T2DM mice | Plasma | LC-MS | Carbohydrates, lipids, and amino acids | Fructose and mannose metabolism, galactose metabolism, arachidonic acid metabolism, TCA cycle, glycerolipid metabolism, sphingolipid metabolism, glycerophospholipid metabolism, and amino acid metabolism | [73] |
| Dendrobium officinale stem (DOP) | T2DM rats | Liver | LC-MS | Cholic acid, deoxycholic acid, glycerophospholipids, lysophosphatidylcholine (LPC), and phosphatidylethanolamines | Fatty acid, glycerolipid, glycerophospholipid, ceramide, and bile acids metabolism | [74] |
| Litchi chinensis Sonn (LSE) | T2DM rats | Serum and urine | LC-MS GC-MS |
Carbohydrate, organic acids, amino acids, etc. | Serine, glycine, arginine, threonine, proline, alanine metabolism, etc. | [75] |
TCM, either alone or in combination, induces changes in a broad spectrum of metabolites. Huanglian Decoction (HLD), a traditional TCM formulation used for centuries, was investigated for its therapeutic effects on T2DM in rats. Urinary metabolomics revealed biomarkers associated with glyoxylate and phenylalanine metabolism, dicarboxylate metabolism, and the tricarboxylic acid (TCA) cycle, providing dynamic insights into HLD’s therapeutic impact.63 Another TCM preparation, Hypoglycemic decoction (HD), exhibited significant therapeutic effects on T2DM. Metabolomics analysis identified potential biomarkers linked to phenylalanine metabolism, TCA cycle, glyoxylate metabolism, and dicarboxylate metabolism.71
Dendrobium officinale polysaccharide (DOP), a key ingredient in D. officinale with metabolism-modulatory activities in T2DM, was studied for its impact on liver lipidomics and metabolomics. The results indicated that DOP can modulate glycerolipid, fatty acid, glycerophospholipid, ceramide, and bile acid metabolism, presenting a potential avenue for managing T2DM.74 Litchi chinensis Sonn. seed extract (LSE), commonly used in TCM to mitigate diabetic risk factors, demonstrated its hypoglycemic mechanism through broad-spectrum metabolic changes in T2DM rats.75
Different TCMs chosen in the same stage of T2DM disease caused a similar metabolic response. Whether these pathways or metabolites constitute a shared mechanism for various therapies is unknown. Understanding this may help us investigate new T2DM therapeutics or develop new drugs. These studies indicated that metabolomics, one of the most critical systems biology platforms, can identify and characterize the organism’s biochemical responses to TCM. This approach provided a practical method for future interventions and TCM assessments.
Metabolomics Studies of TCM Treating Diabetic Complications
Long-term insulin deficiency leads to macrovascular and microvascular complications and even death in patients with diabetes. Microvascular complications in patients with T2DM causing renal failure, blindness, and non-traumatic amputations, are effective predictors of cardiovascular complications.76 There is no available treatment when the disease is diagnosed, but early treatment at a subclinical level can prevent or at least delay disease progression. Thus, it is vital to identify early biomarkers to inhibit the disease. Metabolomics has been used to classify the metabolic characteristics of T2DM in many different biological systems. Current challenges include linking these biomarkers to specific complications to better predict future risks and disease progression.77
Diabetic complications are usually treated with western medicine or surgery.78–81 However, effective prevention and treatment methods are lacking. Effective medications with fewer side effects need to be discovered. The efficacy and mechanism of TCM on T2DM and diabetic complications have been gradually recognized and clarified. However, the composition of TCM is complex, and it is challenging to elucidate the treatment mechanism. Strategies and methods appropriate for complex system analysis are required to further explain TCM’s mechanism of action and investigate pharmacodynamics’ basis. Considering its advantages, metabonomics has become the research focus to reveal the mechanism of action of TCM.
Diabetic Retinopathy
Diabetic retinopathy (DR), a leading cause of adult blindness globally, stems from alterations in retinal microvasculature.82 Proliferative diabetic retinopathy (PDR), characterized by retinal neovascularization (NV), is a common cause of vision loss in diabetic patients. Metabolomics-based studies on DR biomarkers have gained interest (Table 3).
Table 3.
Biomarker Discovery in Diabetic Retinopathy
| Prediction | Detective Method | Metabolic Biomarkers | Ref. |
|---|---|---|---|
| Proliferative diabetic retinopathy (PDR) | LC-MS | Pyruvate, lactate, proline, allantoin, creatine | [83] |
| DR | LC-MS | Total DMA, tryptophan, and kynurenine | [84] |
| PDR | LC-MS | Inosine, hypoxanthine, urate, allantoate | [85] |
| DR | GC‐MSM, LC‐MSM, and LC‐MSL | 2-piperidone and 12-hydroxyeicosatetraenoic acid (12-HETE) | [86] |
| PDR | LC-MS | Fumaric acid, uridine, acetic acid, and cytidine | [87] |
| DR | LC-MS | Arginine, citrulline, glutamic γ-semialdehyde, and dehydroxycarnitine | [88] |
| PDR | LC-MS | Arginine and Carnitine | [88] |
| DR | 1 H-NMR | Lactate, succinate, 2-hydroxybutyrate, asparagine, dimethylamine, histidine, threonine, and glutamine | [89] |
| DR | GC-MS | Threonic acid, d-2,3-Dihydroxypropanoic acid, l-Lactic acid, isocitric acid, fructose 6-phosphate, l-threonine, ornithine, l-glutamine, pyroglutamic acid, pyruvic acid, and l-alanine | [90] |
| DR | GC-MS, LC-MS | Plasma glutamine, glutamic acid, and their ratio | [91] |
| DR | LC-MS | FAS, LPCs, LPC-Os, LPEs, LPE-ps, Cers, CerG1s, etc | [92] |
Tomita et al investigated DR metabolites in vitreous samples from patients with PDR, identifying 158 potential biomarkers associated with the anti-DR effect, including pyruvate, lactate, proline, allantoin, and creatine. Creatine supplementation may inhibit NV in PDR, suggesting its potential therapeutic benefit.83 Another study by Xuan et al conducted serum metabolic profiling in diabetic patients without DR (NDR) and with different stages of DR. Biomarkers, such as 12-hydroxyeicosatetraenoic acid (12-HETE) and 2-piperidone, outperformed hemoglobin A1c (HbA1c) in differentiating DR from diabetes and detecting early-stage DR.86
While most research focuses on discovering early biomarkers for inhibiting DR progression, there’s a gap in understanding the metabolomic mechanisms of traditional Chinese medicine (TCM) effects. Bushen Huoxue Prescription (BP), comprising Salviae Miltiorrhizae Radix et Rhizoma, Rehmanniae Radix, Puerariae Lobatae Radix, and Ginseng Radix et Rhizoma, has shown promise in DR prevention and treatment. Using urine metabolomics, BP’s anti-DR effect was linked to nine small-molecule metabolites, indicating its potential to regulate gut microbial metabolism, lipid metabolism, and tryptophan metabolism in treating DR.93 These findings underscore the need for more research to elucidate the molecular effects of TCM on DR’s physiological changes.
Diabetic Nephropathy
Diabetic nephropathy (DN), characterized by decreased glomerular filtration rate and increased urinary albumin excretion, poses a significant risk,94 affecting about 30% of diabetic patients and potentially progressing to end-stage kidney disease. The associated cardiovascular death risk is notably higher than in individuals with normal renal function.95 Table 4 summarizes the metabolomics of DN patients.
Table 4.
Biomarker Discovery in Diabetic Nephropathy
| Prediction | Detective Method | Metabolic Biomarkers | Ref. |
|---|---|---|---|
| DN | GC-MS | Serum: benzoic acid, glycerol 1-octadecanoate, fumaric acid, erythrose, L-glutamic acid/pyroglutamic acid, fructose 6-phophate, L-Arabitol, taurine, and L-glutamine. Urine: D-glucose, gluconic acid, L-histidine, L-valine, sucrose, glycine, L-xylonate-2, L-asparagine/L-aspartic acid, and oxalic acid. |
[96] |
| DN | LC-MS | Tyrosine | [97] |
| DN | LC-MS | Linolelaidic Acid (C18:2N6T), 6-Aminocaproic Acid, Hexadecanoic Acid (C16:0), Trans-4-Hydroxy-L-Proline, L-Dihydroorotic Acid, Azoxystrobin Acid, Linoleic Acid (C18:2N6C), 6-Methylmercaptopurine, Lysopc 20:4, Piperidine, and Cuminaldehyde | [98] |
| DN | NMR | Glycoprotein acetyls, branched-chain and aromatic amino acids (AAAs), triglycerides (TGs), lipids in very low-density lipoproteins (VLDL), cholesterol, fatty acids, and phospholipids in high-density lipoproteins (HDL) and apolipoprotein A1. | [99] |
| DN | LC-MS | 3-methylcrotonyglycine, 3-hydroxyisobutyrate (3-HIBA), citric and aconitic acid | [100] |
| DN | LC-MS | Pantothenic acid | [101] |
Existing literature and recent studies have identified potential metabolites for monitoring DN.102 Representative studies include Zhang et al, who used gas chromatography coupled with time-of-flight mass spectrometry to identify serum and urine metabolites distinguishing DN from T2DM.96 Tofte et al utilized nuclear magnetic resonance spectroscopy, linking 125 metabolites strongly to estimated glomerular filtration rate, revealing associations with glycoprotein acetyls, amino acids, triglycerides, lipids, and fatty acids.99
Metabolomics has also been applied to TCM research in addressing diabetic nephropathy issues.103–105 Notably, Salvia miltiorrhiza Bunge (SM) has shown therapeutic effects on various T2DM complications, including diabetic nephropathy. Xiang et al investigated the metabolic changes induced by SM in plasma, urine, and renal tissues of DN rats. SM extracts alleviated renal injury and regulated glycolipid metabolism, triggering significant metabolic alterations in serum, urine, and kidney tissues. This metabolic network, including phospholipid, arachidonic acid, and pyrimidine metabolisms, supports the protective effects of SM in DN rats.106–109
While initial studies demonstrate the potential of metabolomics in understanding DN’s metabolic patterns, development, and differentiation, further research is necessary for a more accurate analysis.
Diabetic Peripheral Neuropathy
Diabetic peripheral neuropathy (DPN), a common complication of pre-diabetes and T2DM, significantly contributes to lower-limb amputation and neuropathic pain.110 Recent metabolomic studies on DPN patients have highlighted abnormal concentrations of glucosamine,111 1-deoxydihydroceramides,112 and sphingosine113 in sciatic nerves. A meta-analysis emphasized the correlation between histidine, pyruvate, alanine, tyrosine, leucine, and valine levels in cerebrospinal fluid and plasma with the presence of DPN, shedding light on potential metabolic disorders114 (Table 5).
Table 5.
Biomarker Discovery in Peripheral Neuropathy
TCM, with its rich bioactive compounds and holistic approach, presents a promising avenue for addressing DPN. Jin-Mai-Tong (JMT) decoction, consisting of 12 diverse components, has been studied for its neuroprotective effect on diabetic peripheral neuropathy rats.115,116 Metabolomics revealed 21 potential biomarkers associated with JMT’s therapeutic effect, primarily involving the tricarboxylic acid (TCA) cycle, lipid metabolism, and amino acid metabolism. This suggests that JMT decoction improves the metabolism of diabetic rats with peripheral neuropathy, countering DPN.117
Despite increasing attention to diabetic complications, the underlying mechanisms remain unclear. Prospective human sample studies and investigations into diabetic complications within metabonomics research are still limited. Identifying potential biomarkers for T2DM and its complications holds promise for prediction and prevention. While TCM’s efficacy in treating microvascular complications has been explored, the metabolic response to these complications is seldom reported in metabolomic studies. Utilizing metabolomics to delve into TCM mechanisms in humans, although in its early stages, has the potential to inform clinical practice and drug discovery. Future research should extend beyond isolated pharmacodynamic studies, establishing robust connections between symptom improvement and metabolome restoration.
Prospects: Potential of Multi-Omics and Microbiome Integration in TCM Treating T2DM
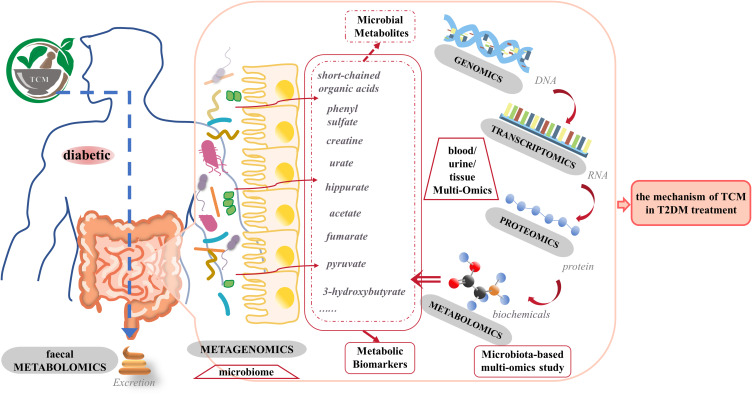
While metabonomics provides insights into metabolites and pathways, its capacity to fully unravel the therapeutic mechanism of TCM for T2DM is limited. To address this, integrating omics and systems biology techniques with traditional methods is imperative (Figure 1).
Figure 1.
Application of Multi-Omics and microbiome Integration in TCM Treating T2DM. The human microbiome plays a role in the etiology and pathology of T2DM and its complications. Metabolites originating from microorganisms are potentially important compounds that mediate microbiota-host interactions in health and disease. External factors such as TCM and internal factors such as the host genome can influence microbial diversity. Changes in the microbiome later affect the metabolites that they produce. Metagenomics is suitable for determining intestinal microbiota composition, while metabolomics can find functional endpoints. Genomics obtained candidate genes related to transcriptomics, while transcriptomics obtained functional gene clusters related to disease pathogenesis. Proteomics can analyze expressed proteins and protein function in a cellular context. It will be vital to reveal the role of the microbiome in T2DM and the mechanism of traditional Chinese medicine in T2DM treatment by integrating metagenomic data and data generated by host omics in the future.
Omics technologies—genomics, proteomics, transcriptomics, and metabolomics—serve as invaluable tools for studying biological systems, especially in the TCM context.118,119 A comprehensive understanding necessitates merging data from diverse omics approaches. Metabolomics complements other omics data, forming a foundational understanding via gene-transcript-protein-metabolite profiles in human tissues,120–123 elucidating the mechanism of TCM treatment for T2DM and identifying therapeutic targets.
Key biomarkers in TCM treatment for T2DM, such as acetate, organic acids, pyruvate, and others, reflect endogenous metabolites influenced by both the host and commensal microorganisms.124–127 TCM induces significant shifts in gut microbiota,128 particularly in the gastrointestinal microflora, influencing T2DM etiology and pathology.129–132
Host genomics, pivotal in microbiome composition determination,133 can be effectively studied through metagenomics, which collectively examines microbial genomes. Integrated with host transcriptome analysis, this approach reveals molecular mechanisms and the impact of microbial variation on gene expression. Proteomics, complementing metagenomics, offers a detailed analysis of microbiome structure and function, illustrating its relationship with the human body.134 The functional influence of the microbiome on metabolic pathways explains its significant association with disease.
In summary, understanding the intricate interactions between the host’s multi-omics and microbiome provides novel insights into TCM’s efficacy in treating T2DM.135,136 The absence of a systematic approach to integrate TCM, microbiome, and in-depth multi-omics in T2DM patients unveils opportunities for studying human wellness and disease. The integration of multi-omics and microbiomes holds promise in developing individualized medicine for T2DM, representing a crucial research direction for the future of TCM in T2DM treatment.
Conclusion
Early management of T2DM is pivotal for averting complications and mortality. Despite advancements in prevention and therapy, persistent diabetic complications underscore the need for novel therapeutic targets. Metabonomics, employing a top-down strategy, systematically delineates body function and detects holistic metabolic changes. By quantifying circulating metabolites across pathways, this approach facilitates early identification of high-risk individuals. Metabolomics provides profound insights into T2DM pathogenesis, treatment, and the mechanisms of TCM. As metabolomics advances, TCM modernization progresses, revealing biomarkers for diverse T2DM types and stages. Strengthening TCM modernization studies is imperative. The metabonomics strategy emerges as a potent tool for exploring TCM’s therapeutic basis, promising significant strides in advancing T2DM diagnosis, prevention, and treatment, thereby offering vital guidance for clinical practice.
Funding Statement
This review was funded by the Science and Technology Research Project of the Education Department of Jilin Province (Under grant No. JJKH20210989KJ) and the Traditional Chinese Medicine Research Project in Qingdao (Under grant No. 2019-zyy033).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/s0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi: 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- 5.Meng X, Liu X, Tan J, et al. From Xiaoke to diabetes mellitus: a review of the research progress in traditional Chinese medicine for diabetes mellitus treatment. Chin Med. 2023;18(1):75. doi: 10.1186/s13020-023-00783-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan KW, Kwong ASK, Tan KCB, et al. Add-on rehmannia-6-based Chinese medicine in type 2 diabetes and CKD: a multicenter randomized controlled trial. Clin J Am Soc Nephrol. 2023;18(9):1163–1174. doi: 10.2215/cjn.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YK, Liu TT, Teia FKF, Xie MZ. Exploring the underlying mechanisms of obesity and diabetes and the potential of traditional Chinese medicine: an overview of the literature. Front Endocrinol. 2023;14:1218880. doi: 10.3389/fendo.2023.1218880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro MS, Carvalho M, Bastos ML, Guedes de Pinho P. Metabolomics analysis for biomarker discovery: advances and challenges. Curr Med Chem. 2013;20(2):257–271. doi: 10.2174/092986713804806621 [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Chen L, Liu D, Chen H, Tang DD, Zhao YY. Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem Biol Interact. 2017;273:133–141. doi: 10.1016/j.cbi.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 10.Ren JL, Zhang AH, Kong L, et al. Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine. 2020;67:153165. doi: 10.1016/j.phymed.2019.153165 [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Sun H, Zhang A, Sun W, Wang P, Wang Z. Potential role of metabolomics apporoaches in the area of traditional Chinese medicine: as pillars of the bridge between Chinese and Western medicine. J Pharm Biomed Anal. 2011;55(5):859–868. doi: 10.1016/j.jpba.2011.01.042 [DOI] [PubMed] [Google Scholar]
- 12.Zhang A, Sun H, Wang Z, Sun W, Wang P, Wang X. Metabolomics: towards understanding traditional Chinese medicine. Planta Med. 2010;76(17):2026–2035. doi: 10.1055/s-0030-1250542 [DOI] [PubMed] [Google Scholar]
- 13.Tong XL, Dong L, Chen L, Zhen Z. Treatment of diabetes using traditional Chinese medicine: past, present and future. Am J Chin Med. 2012;40(5):877–886. doi: 10.1142/s0192415x12500656 [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Yang M, Liu T, Yang L, Ji G. A metabolomics approach to stratify patients diagnosed with diabetes mellitus into excess or deficiency syndromes. Evid Based Complement Alternat Med. 2015;2015:350703. doi: 10.1155/2015/350703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun DZ, Li SD, Liu Y, Zhang Y, Mei R, Yang MH. Differences in the origin of philosophy between Chinese medicine and western medicine: exploration of the holistic advantages of Chinese medicine. Chin J Integr Med. 2013;19(9):706–711. doi: 10.1007/s11655-013-1435-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Ma Q, Li Y, et al. Research progress on traditional Chinese medicine syndromes of diabetes mellitus. Biomed Pharmacother. 2020;121:109565. doi: 10.1016/j.biopha.2019.109565 [DOI] [PubMed] [Google Scholar]
- 17.Schnyer RN, Citkovitz C. Inter-rater reliability in traditional Chinese Medicine: challenging paradigmatic assumptions. J Altern Complement Med. 2019;25(11):1067–1073. doi: 10.1089/acm.2019.0331 [DOI] [PubMed] [Google Scholar]
- 18.Schnyer RN, McKnight P, Conboy LA, et al. Can reliability of the Chinese medicine diagnostic process be improved? Results of a prospective randomized controlled trial. J Altern Complement Med. 2019;25(11):1103–1108. doi: 10.1089/acm.2019.0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu GS, Li HK, Zhang WD. Metabolomics and its application in the treatment of coronary heart disease with traditional Chinese medicine. Chin J Nat Med. 2019;17(5):321–330. doi: 10.1016/s1875-5364(19)30037-8 [DOI] [PubMed] [Google Scholar]
- 20.Sun H, Zhang A, Wang X. Potential role of metabolomic approaches for Chinese medicine syndromes and herbal medicine. Phytother Res. 2012;26(10):1466–1471. doi: 10.1002/ptr.4613 [DOI] [PubMed] [Google Scholar]
- 21.Jiang N, Liu HF, Li SD, et al. An integrated metabonomic and proteomic study on Kidney-Yin deficiency syndrome patients with diabetes mellitus in China. Acta Pharmacol Sin. 2015;36(6):689–698. doi: 10.1038/aps.2014.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care. 2011;34 Suppl 2(Suppl 2):S202–S209. doi: 10.2337/dc11-s221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beulens J, Rutters F, Rydén L, et al. Risk and management of pre-diabetes. Eur J Prev Cardiol. 2019;26(2_suppl):47–54. doi: 10.1177/2047487319880041 [DOI] [PubMed] [Google Scholar]
- 24.Wishart DS. Metabolomics for investigating physiological and pathophysiological processes. Physiological Rev. 2019;99(4):1819–1875. doi: 10.1152/physrev.00035.2018 [DOI] [PubMed] [Google Scholar]
- 25.Jacob M, Lopata AL, Dasouki M, Abdel Rahman AM. Metabolomics toward personalized medicine. Mass Spectrom Rev. 2019;38(3):221–238. doi: 10.1002/mas.21548 [DOI] [PubMed] [Google Scholar]
- 26.Jun G, Aguilar D, Evans C, Burant CF, Hanis CL. Metabolomic profiles associated with subtypes of prediabetes among Mexican Americans in Starr County, Texas, USA. Diabetologia. 2020;63(2):287–295. doi: 10.1007/s00125-019-05031-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Zhang Y, Liu X, et al. Metabolite triplet in serum improves the diagnostic accuracy of prediabetes and diabetes screening. J Proteome Res. 2021;20(1):1005–1014. doi: 10.1021/acs.jproteome.0c00786 [DOI] [PubMed] [Google Scholar]
- 28.Fikri AM, Smyth R, Kumar V, Al-Abadla Z, Abusnana S, Munday MR. Pre-diagnostic biomarkers of type 2 diabetes identified in the UAE’s obese national population using targeted metabolomics. Sci Rep. 2020;10(1):17616. doi: 10.1038/s41598-020-73384-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bos MM, Noordam R, Bennett K, et al. Metabolomics analyses in non-diabetic middle-aged individuals reveal metabolites impacting early glucose disturbances and insulin sensitivity. Metabolomics. 2020;16(3):35. doi: 10.1007/s11306-020-01653-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun H, Sun L, Wu Q, et al. Associations among circulating sphingolipids, β-cell function, and risk of developing type 2 diabetes: a population-based cohort study in China. PLoS Med. 2020;17(12):e1003451. doi: 10.1371/journal.pmed.1003451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ottosson F, Smith E, Gallo W, Fernandez C, Melander O. Purine metabolites and carnitine biosynthesis intermediates are biomarkers for incident type 2 diabetes. J Clin Endocrinol Metab. 2019;104(10):4921–4930. doi: 10.1210/jc.2019-00822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen GC, Chai JC, Yu B, et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr. 2020;112(1):57–65. doi: 10.1093/ajcn/nqaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell JA, Bull CJ, Gunter MJ, et al. Early metabolic features of genetic liability to type 2 diabetes: cohort study with repeated metabolomics across early life. Diabetes Care. 2020;43(7):1537–1545. doi: 10.2337/dc19-2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vangipurapu J, Stancáková A, Smith U, Kuusisto J, Laakso M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5181 Finnish men. Diabetes. 2019;68(6):1353–1358. doi: 10.2337/db18-1076 [DOI] [PubMed] [Google Scholar]
- 35.Li L, Krznar P, Erban A, et al. Metabolomics identifies a biomarker revealing in vivo loss of functional β-cell mass before diabetes onset. Diabetes. 2019;68(12):2272–2286. doi: 10.2337/db19-0131 [DOI] [PubMed] [Google Scholar]
- 36.Lu J, Lam SM, Wan Q, et al. High-coverage targeted lipidomics reveals novel serum lipid predictors and lipid pathway dysregulation antecedent to type 2 diabetes onset in normoglycemic Chinese adults. Diabetes Care. 2019;42(11):2117–2126. doi: 10.2337/dc19-0100 [DOI] [PubMed] [Google Scholar]
- 37.Gu X, Al Dubayee M, Alshahrani A, et al. Distinctive metabolomics patterns associated with insulin resistance and type 2 diabetes mellitus. Front Mol Biosci. 2020;7:609806. doi: 10.3389/fmolb.2020.609806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamanti K, Cavalli M, Pan G, et al. Intra- and inter-individual metabolic profiling highlights carnitine and lysophosphatidylcholine pathways as key molecular defects in type 2 diabetes. Sci Rep. 2019;9(1):9653. doi: 10.1038/s41598-019-45906-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mack CI, Ferrario PG, Weinert CH, et al. Exploring the diversity of sugar compounds in healthy, prediabetic, and diabetic volunteers. Mol Nutr Food Res. 2020;64(9):e1901190. doi: 10.1002/mnfr.201901190 [DOI] [PubMed] [Google Scholar]
- 40.Calvani R, Rodriguez-Mañas L, Picca A, et al. Identification of a circulating amino acid signature in frail older persons with type 2 diabetes mellitus: results from the Metabofrail Study. Nutrients. 2020;12(1):199. doi: 10.3390/nu12010199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora-Ortiz M, Nuñez Ramos P, Oregioni A, Claus SP. NMR metabolomics identifies over 60 biomarkers associated with Type II diabetes impairment in db/db mice. Metabolomics. 2019;15(6):89. doi: 10.1007/s11306-019-1548-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godzien J, Kalaska B, Adamska-Patruno E, et al. Oxidized glycerophosphatidylcholines in diabetes through non-targeted metabolomics: their annotation and biological meaning. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1120:62–70. doi: 10.1016/j.jchromb.2019.04.053 [DOI] [PubMed] [Google Scholar]
- 43.Al-Sulaiti H, Diboun I, Agha MV, et al. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J Transl Med. 2019;17(1):348. doi: 10.1186/s12967-019-2096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji Y, Yao Y, Duan Y, et al. Association between urinary organophosphate flame retardant diesters and steroid hormones: a metabolomic study on type 2 diabetes mellitus cases and controls. Sci Total Environ. 2021;756:143836. doi: 10.1016/j.scitotenv.2020.143836 [DOI] [PubMed] [Google Scholar]
- 45.Ottosson F, Smith E, Fernandez C, Melander O. Plasma metabolites associate with all-cause mortality in individuals with type 2 diabetes. Metabolites. 2020;10(8):315. doi: 10.3390/metabo10080315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salihovic S, Broeckling CD, Ganna A, et al. Non-targeted urine metabolomics and associations with prevalent and incident type 2 diabetes. Sci Rep. 2020;10(1):16474. doi: 10.1038/s41598-020-72456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahola-Olli AV, Mustelin L, Kalimeri M, et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019;62(12):2298–2309. doi: 10.1007/s00125-019-05001-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng Y, Mtintsilana A, Goedecke JH, Micklesfield LK, Olsson T, Chorell E. Alterations in the metabolism of phospholipids, bile acids and branched-chain amino acids predicts development of type 2 diabetes in black South African women: a prospective cohort study. Metabolism. 2019;95:57–64. doi: 10.1016/j.metabol.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 49.Gar C, Rottenkolber M, Prehn C, Adamski J, Seissler J, Lechner A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit Rev Clin Lab Sci. 2018;55(1):21–32. doi: 10.1080/10408363.2017.1414143 [DOI] [PubMed] [Google Scholar]
- 50.Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care. 2016;39(5):833–846. doi: 10.2337/dc15-2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein MS, Shearer J. Metabolomics and type 2 diabetes: translating basic research into clinical application. J Diabetes Res. 2016;2016:3898502. doi: 10.1155/2016/3898502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drogan D, Dunn WB, Lin W, et al. Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clin Chem. 2015;61(3):487–497. doi: 10.1373/clinchem.2014.228965 [DOI] [PubMed] [Google Scholar]
- 53.Nikiforova VJ, Giesbertz P, Wiemer J, et al. Glyoxylate, a new marker metabolite of type 2 diabetes. J Diabetes Res. 2014;2014:685204. doi: 10.1155/2014/685204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sas KM, Karnovsky A, Michailidis G, Pennathur S. Metabolomics and diabetes: analytical and computational approaches. Diabetes. 2015;64(3):718–732. doi: 10.2337/db14-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, Gao HY, Fan ZY, He Y, Yan YX. Metabolomics signatures in type 2 diabetes: a systematic review and integrative analysis. J Clin Endocrinol Metab. 2020;105(4):1000–1008. doi: 10.1210/clinem/dgz240 [DOI] [PubMed] [Google Scholar]
- 56.Jia W. Diabetes research in China: making progress. Lancet Diabetes Endocrinol. 2017;5(1):9–10. doi: 10.1016/s2213-8587(16)30094-8 [DOI] [PubMed] [Google Scholar]
- 57.Tian J, Jin D, Bao Q, et al. Evidence and potential mechanisms of traditional Chinese medicine for the treatment of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obesity Metab. 2019;21(8):1801–1816. doi: 10.1111/dom.13760 [DOI] [PubMed] [Google Scholar]
- 58.Luo M, Zhang Z, Lu Y, et al. Urine metabolomics reveals biomarkers and the underlying pathogenesis of diabetic kidney disease. Int Urol Nephrol. 2023;55(4):1001–1013. doi: 10.1007/s11255-022-03326-x [DOI] [PubMed] [Google Scholar]
- 59.Zou J, Xiang Q, Tan D, et al. Zuogui-Jiangtang-Qinggan-Fang alleviates high-fat diet-induced type 2 diabetes mellitus with non-alcoholic fatty liver disease by modulating gut microbiome-metabolites-short chain fatty acid composition. Biomed Pharmacother. 2023;157:114002. doi: 10.1016/j.biopha.2022.114002 [DOI] [PubMed] [Google Scholar]
- 60.Guo S, Qiu S, Cai Y, et al. Mass spectrometry-based metabolomics for discovering active ingredients and exploring action mechanism of herbal medicine. Front Chem. 2023;11:1142287. doi: 10.3389/fchem.2023.1142287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin L, Zhang S, Lin Y, et al. Untargeted metabolomics analysis on Cicer arietinium L.-induced amelioration in T2D rats by UPLC-Q-TOF-MS/MS. J Ethnopharmacol. 2020;261:113013. doi: 10.1016/j.jep.2020.113013 [DOI] [PubMed] [Google Scholar]
- 62.Yan Z, Wu H, Zhou H, et al. Integrated metabolomics and gut microbiome to the effects and mechanisms of naoxintong capsule on type 2 diabetes in rats. Sci Rep. 2020;10(1):10829. doi: 10.1038/s41598-020-67362-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan L, Li Z, Wang Y, Zhang B, Liu G, Liu J. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian Decoction in rats with type 2 diabetes mellitus. J Ethnopharmacol. 2020;258:112842. doi: 10.1016/j.jep.2020.112842 [DOI] [PubMed] [Google Scholar]
- 64.Guo Q, Niu W, Li X, et al. Study on hypoglycemic effect of the drug pair of astragalus radix and dioscoreae rhizoma in T2DM rats by network pharmacology and metabonomics. Molecules. 2019;24(22):4050. doi: 10.3390/molecules24224050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weng J, Zhou J, Liang L, Li L. UHPLC/QTOF-MS-based metabolomics reveal the effect of melastoma dodecandrum extract in type 2 diabetic rats. Pharm Biol. 2019;57(1):807–815. doi: 10.1080/13880209.2019.1693605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Yang C, Song F, Liu Z, Liu S. Therapeutic effectiveness of gardenia jasminoides on type 2 diabetic rats: mass spectrometry-based metabolomics approach. Journal of Agricultural and Food Chemistry. 2020;68(36):9673–9682. doi: 10.1021/acs.jafc.0c02873 [DOI] [PubMed] [Google Scholar]
- 67.Wang H, Huang R, Li H, Jiao L, Liu S, Wu W. Serum metabolomic analysis of the anti-diabetic effect of Ginseng berry in type II diabetic rats based on ultra high-performance liquid chromatography-high resolution mass spectrometry. J Pharm Biomed Anal. 2021;196:113897. doi: 10.1016/j.jpba.2021.113897 [DOI] [PubMed] [Google Scholar]
- 68.Li YY, Stewart DA, Ye XM, et al. A metabolomics approach to investigate kukoamine B-A potent natural product with anti-diabetic properties. Front Pharmacol. 2018;9:1575. doi: 10.3389/fphar.2018.01575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu F, Zhang YQ. Metabolic effects of mulberry branch bark powder on diabetic mice based on GC-MS metabolomics approach. Nutr Metab. 2019;16:10. doi: 10.1186/s12986-019-0335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng J, Guo Y, Hu B, et al. Serum metabolomic profiles reveal the impact of BuZangTongLuo formula on metabolic pathways in diabetic mice with hindlimb ischemia. J Ethnopharmacol. 2020;258:112928. doi: 10.1016/j.jep.2020.112928 [DOI] [PubMed] [Google Scholar]
- 71.Pan LL, Sun QH, Liu GR, Guo JY. Urinary Metabolomics Study of the intervention effect of hypoglycemic decoction on type 2 diabetes mellitus rats model. Evid Based Complement Alternat Med. 2019;2019:1394641. doi: 10.1155/2019/1394641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He WJ, Cao DM, Chen YB, et al. Explore of the beneficial effects of Huang-Lian-Jie-Du Decoction on diabetic encephalopathy in db/db mice by UPLC-Q-Orbitrap HRMS/MS based untargeted metabolomics analysis. J Pharm Biomed Anal. 2021;192:113652. doi: 10.1016/j.jpba.2020.113652 [DOI] [PubMed] [Google Scholar]
- 73.Xiang Z, Xie H, Tong Q, et al. Revealing hypoglycemic and hypolipidemic mechanism of Xiaokeyinshui extract combination on streptozotocin-induced diabetic mice in high sucrose/high fat diet by metabolomics and lipidomics. Biomed Pharmacother. 2021;135:111219. doi: 10.1016/j.biopha.2021.111219 [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Chen H, Nie Q, Huang X, Nie S. Dendrobium officinale polysaccharide ameliorates the liver metabolism disorders of type II diabetic rats. Int J Biol Macromol. 2020;164:1939–1948. doi: 10.1016/j.ijbiomac.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 75.Man S, Ma J, Yao J, et al. Systemic perturbations of key metabolites in type 2 diabetic rats treated by polyphenol extracts from Litchi chinensis seeds. Journal of Agricultural and Food Chemistry. 2017;65(35):7698–7704. doi: 10.1021/acs.jafc.7b02206 [DOI] [PubMed] [Google Scholar]
- 76.Avogaro A, Fadini GP. Microvascular complications in diabetes: a growing concern for cardiologists. Int J Cardiol. 2019;291:29–35. doi: 10.1016/j.ijcard.2019.02.030 [DOI] [PubMed] [Google Scholar]
- 77.Eid S, Sas KM, Abcouwer SF, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62(9):1539–1549. doi: 10.1007/s00125-019-4959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19(6). doi: 10.3390/ijms19061816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matuszewski W, Baranowska-Jurkun A, Stefanowicz-Rutkowska MM, Gontarz-Nowak K, Gątarska E, Bandurska-Stankiewicz E. The safety of pharmacological and surgical treatment of diabetes in patients with diabetic retinopathy-a review. J Clin Med. 2021;10(4):705. doi: 10.3390/jcm10040705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamazaki T, Mimura I, Tanaka T, Nangaku M. Treatment of diabetic kidney disease: current and future. Diabet Metabol J. 2021;45(1):11–26. doi: 10.4093/dmj.2020.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raghu ALB, Parker T, Aziz TZ, et al. Invasive electrical neuromodulation for the treatment of painful diabetic neuropathy: systematic review and meta-analysis. Neuromodulation. 2021;24(1):13–21. doi: 10.1111/ner.13216 [DOI] [PubMed] [Google Scholar]
- 82.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/s0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 83.Tomita Y, Cagnone G, Fu Z, et al. Vitreous metabolomics profiling of proliferative diabetic retinopathy. Diabetologia. 2021;64(1):70–82. doi: 10.1007/s00125-020-05309-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yun JH, Kim JM, Jeon HJ, Oh T, Choi HJ, Kim BJ. Metabolomics profiles associated with diabetic retinopathy in type 2 diabetes patients. PLoS One. 2020;15(10):e0241365. doi: 10.1371/journal.pone.0241365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haines NR, Manoharan N, Olson JL, D’Alessandro A, Reisz JA. Metabolomics analysis of human vitreous in diabetic retinopathy and rhegmatogenous retinal detachment. J Proteome Res. 2018;17(7):2421–2427. doi: 10.1021/acs.jproteome.8b00169 [DOI] [PubMed] [Google Scholar]
- 86.Xuan Q, Ouyang Y, Wang Y, et al. Multiplatform metabolomics reveals novel serum metabolite biomarkers in diabetic retinopathy subjects. Adv Sci. 2020;7(22):2001714. doi: 10.1002/advs.202001714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu XR, Yang FY, Lu J, et al. Plasma metabolomic profiling of proliferative diabetic retinopathy. Nutr Metab. 2019;16:37. doi: 10.1186/s12986-019-0358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sumarriva K, Uppal K, Ma C, et al. Arginine and carnitine metabolites are altered in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2019;60(8):3119–3126. doi: 10.1167/iovs.19-27321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin H, Zhu B, Liu X, Jin J, Zou H. Metabolic characterization of diabetic retinopathy: an (1)H-NMR-based metabolomic approach using human aqueous humor. J Pharm Biomed Anal. 2019;174:414–421. doi: 10.1016/j.jpba.2019.06.013 [DOI] [PubMed] [Google Scholar]
- 90.Wang H, Fang J, Chen F, et al. Metabolomic profile of diabetic retinopathy: a GC-TOFMS-based approach using vitreous and aqueous humor. Acta Diabetol. 2020;57(1):41–51. doi: 10.1007/s00592-019-01363-0 [DOI] [PubMed] [Google Scholar]
- 91.Rhee SY, Jung ES, Park HM, et al. Plasma glutamine and glutamic acid are potential biomarkers for predicting diabetic retinopathy. Metabolomics. 2018;14(7):89. doi: 10.1007/s11306-018-1383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xuan Q, Zheng F, Yu D, et al. Rapid lipidomic profiling based on ultra-high performance liquid chromatography-mass spectrometry and its application in diabetic retinopathy. Anal Bioanal Chem. 2020;412(15):3585–3594. doi: 10.1007/s00216-020-02632-6 [DOI] [PubMed] [Google Scholar]
- 93.Wang X, Li Y, Xie M, Deng L, Zhang M, Xie X. Urine metabolomics study of Bushen Huoxue prescription on diabetic retinopathy rats by UPLC-Q-exactive orbitrap-MS. Biomed Chromatogr. 2020;34(4):e4792. doi: 10.1002/bmc.4792 [DOI] [PubMed] [Google Scholar]
- 94.Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets. 2019;23(7):579–591. doi: 10.1080/14728222.2019.1624721 [DOI] [PubMed] [Google Scholar]
- 95.Maqbool M, Cooper ME, Jandeleit-Dahm KAM. Cardiovascular disease and diabetic kidney disease. Semin Nephrol. 2018;38(3):217–232. doi: 10.1016/j.semnephrol.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 96.Shao M, Lu H, Yang M, et al. Serum and urine metabolomics reveal potential biomarkers of T2DM patients with nephropathy. Ann Transl Med. 2020;8(5):199. doi: 10.21037/atm.2020.01.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang S, Li X, Luo H, Fang ZZ, Ai H. Role of aromatic amino acids in pathogeneses of diabetic nephropathy in Chinese patients with type 2 diabetes. J diabet complicat. 2020;34(10):107667. doi: 10.1016/j.jdiacomp.2020.107667 [DOI] [PubMed] [Google Scholar]
- 98.Zhang H, Zuo JJ, Dong SS, et al. Identification of potential serum metabolic biomarkers of diabetic kidney disease: a Widely Targeted Metabolomics Study. J Diabetes Res. 2020;2020:3049098. doi: 10.1155/2020/3049098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tofte N, Vogelzangs N, Mook-Kanamori D, et al. Plasma metabolomics identifies markers of impaired renal function: a meta-analysis of 3089 persons with type 2 diabetes. J Clin Endocrinol Metab. 2020;105(7):2275–2287. doi: 10.1210/clinem/dgaa173 [DOI] [PubMed] [Google Scholar]
- 100.Kwan B, Fuhrer T, Zhang J, et al. Metabolomic markers of kidney function decline in patients with diabetes: evidence from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2020;76(4):511–520. doi: 10.1053/j.ajkd.2020.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma T, Liu T, Xie P, et al. UPLC-MS-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and CoA biosynthesis pathway in diabetic kidney disease. Life Sci. 2020;258:118160. doi: 10.1016/j.lfs.2020.118160 [DOI] [PubMed] [Google Scholar]
- 102.Cordero-Pérez P, Sánchez-Martínez C, García-Hernández PA, Saucedo AL. [Metabolomics of the diabetic nephropathy: behind the fingerprint of development and progression indicators] Metabolómica de la nefropatía diabética: tras la huella de indicadores de desarrollo y progresión. Nefrologia. 2020;40(6):585–596. Spanish. doi: 10.1016/j.nefro.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 103.Meng X, Ma J, Kang AN, Kang SY, Jung HW, Park YK. A novel approach based on metabolomics coupled with intestinal flora analysis and network pharmacology to explain the mechanisms of action of bekhogainsam decoction in the improvement of symptoms of streptozotocin-induced diabetic nephropathy in mice. Front Pharmacol. 2020;11:633. doi: 10.3389/fphar.2020.00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, He Q, Chen Q, et al. Network pharmacology combined with metabolomics to study the mechanism of Shenyan Kangfu tablets in the treatment of diabetic nephropathy. J Ethnopharmacol. 2021;270:113817. doi: 10.1016/j.jep.2021.113817 [DOI] [PubMed] [Google Scholar]
- 105.Du Y, Xu BJ, Deng X, et al. Predictive metabolic signatures for the occurrence and development of diabetic nephropathy and the intervention of Ginkgo biloba leaves extract based on gas or liquid chromatography with mass spectrometry. J Pharm Biomed Anal. 2019;166:30–39. doi: 10.1016/j.jpba.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 106.Guo JC, Pan HC, Yeh BY, et al. Associations between using Chinese herbal medicine and long-term outcome among pre-dialysis diabetic nephropathy patients: a Retrospective Population-Based Cohort Study. Front Pharmacol. 2021;12:616522. doi: 10.3389/fphar.2021.616522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li CL, Liu B, Wang ZY, et al. Salvianolic acid B improves myocardial function in diabetic cardiomyopathy by suppressing IGFBP3. J Mol Cell Cardiol. 2020;139:98–112. doi: 10.1016/j.yjmcc.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 108.Yin Z, Wang X, Yang X, Chen Y, Duan Y, Han J. Salvia miltiorrhiza in anti-diabetic angiopathy. Curr Mol Pharmacol. 2021;14(6):960–974. doi: 10.2174/1874467214999210111222918 [DOI] [PubMed] [Google Scholar]
- 109.Xiang X, Cai HD, Su SL, et al. Salvia miltiorrhiza protects against diabetic nephropathy through metabolome regulation and wnt/β-catenin and TGF-β signaling inhibition. Pharmacol Res. 2019;139:26–40. doi: 10.1016/j.phrs.2018.10.030 [DOI] [PubMed] [Google Scholar]
- 110.Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938–948. doi: 10.1016/s2213-8587(19)30081-6 [DOI] [PubMed] [Google Scholar]
- 111.Mizukami H, Osonoi S, Takaku S, et al. Role of glucosamine in development of diabetic neuropathy independent of the aldose reductase pathway. Brain Comm. 2020;2(2):fcaa168. doi: 10.1093/braincomms/fcaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fridman V, Zarini S, Sillau S, et al. Altered plasma serine and 1-deoxydihydroceramide profiles are associated with diabetic neuropathy in type 2 diabetes and obesity. J diabet complicat. 2021;35(4):107852. doi: 10.1016/j.jdiacomp.2021.107852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Durán AM, Salto LM, Câmara J, et al. Effects of omega-3 polyunsaturated fatty-acid supplementation on neuropathic pain symptoms and sphingosine levels in Mexican-Americans with type 2 diabetes. Diabetes Metab Syndr Obes. 2019;12:109–120. doi: 10.2147/dmso.S187268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin HT, Cheng ML, Lo CJ, et al. (1)H Nuclear Magnetic Resonance (NMR)-based cerebrospinal fluid and plasma metabolomic analysis in type 2 diabetic patients and risk prediction for diabetic microangiopathy. J Clin Med. 2019;8(6). doi: 10.3390/jcm8060874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang X, Cui L, Guo S. [Clinical study on jinmaitong composita on diabetic peripheral neuropathy]. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi. 1999;19(9):517–519. Chinese. [PubMed] [Google Scholar]
- 116.Song W, Jiang W, Wang C, et al. Jinmaitong, a traditional Chinese compound prescription, ameliorates the streptozocin-induced diabetic peripheral neuropathy rats by increasing sciatic nerve IGF-1 and IGF-1R expression. Front Pharmacol. 2019;10:255. doi: 10.3389/fphar.2019.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Q, Song W, Liang X, et al. A metabolic insight into the neuroprotective effect of Jin-Mai-Tong (JMT) decoction on diabetic rats with peripheral neuropathy using untargeted metabolomics strategy. Front Pharmacol. 2020;11:221. doi: 10.3389/fphar.2020.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng X, Chen T, Jiang R, et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33(4):791–803.e7. doi: 10.1016/j.cmet.2020.11.017 [DOI] [PubMed] [Google Scholar]
- 119.Guo R, Luo X, Liu J, Liu L, Wang X, Lu H. Omics strategies decipher therapeutic discoveries of traditional Chinese medicine against different diseases at multiple layers molecular-level. Pharmacol Res. 2020;152:104627. doi: 10.1016/j.phrs.2020.104627 [DOI] [PubMed] [Google Scholar]
- 120.Sha Q, Lyu J, Zhao M, Li H, Guo M, Sun Q. Multi-omics analysis of diabetic nephropathy reveals potential new mechanisms and drug targets. Front Genetics. 2020;11:616435. doi: 10.3389/fgene.2020.616435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darmayanti S, Lesmana R, Meiliana A, Abdulah R. Genomics, proteomics and metabolomics approaches for predicting diabetic nephropathy in type 2 diabetes mellitus patients. Curr Diabetes Rev. 2020. doi: 10.2174/1573399817666210101105253 [DOI] [PubMed] [Google Scholar]
- 122.Chen D, Zhao X, Sui Z, et al. A multi-omics investigation of the molecular characteristics and classification of six metabolic syndrome relevant diseases. Theranostics. 2020;10(5):2029–2046. doi: 10.7150/thno.41106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen ZZ, Gerszten RE. Metabolomics and proteomics in type 2 diabetes. Circ Res. 2020;126(11):1613–1627. doi: 10.1161/circresaha.120.315898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang M, Lao L. Emerging applications of metabolomics in Traditional chinese medicine treating hypertension: biomarkers, pathways and more. Front Pharmacol. 2019;10:158. doi: 10.3389/fphar.2019.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kikuchi K, Saigusa D, Kanemitsu Y, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10(1):1835. doi: 10.1038/s41467-019-09735-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vangipurapu J, Fernandes Silva L, Kuulasmaa T, Smith U, Laakso M. Microbiota-related metabolites and the risk of type 2 diabetes. Diabetes Care. 2020;43(6):1319–1325. doi: 10.2337/dc19-2533 [DOI] [PubMed] [Google Scholar]
- 127.Hameed A, Mojsak P, Buczynska A, Suleria HAR, Kretowski A, Ciborowski M. Altered metabolome of lipids and amino acids species: a source of early signature biomarkers of T2DM. J Clin Med. 2020;9(7):2257. doi: 10.3390/jcm9072257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li J, Li R, Li N, et al. Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. J Pharm Biomed Anal. 2018;158:451–460. doi: 10.1016/j.jpba.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 129.Thingholm LB, Rühlemann MC, Koch M, et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe. 2019;26(2):252–264.e10. doi: 10.1016/j.chom.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol. 2019;15(2):69–70. doi: 10.1038/s41574-018-0143-9 [DOI] [PubMed] [Google Scholar]
- 131.Zhao L, Lou H, Peng Y, Chen S, Fan L, Li X. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res Clin Pract. 2020;169:108418. doi: 10.1016/j.diabres.2020.108418 [DOI] [PubMed] [Google Scholar]
- 132.Yang G, Wei J, Liu P, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712. doi: 10.1016/j.metabol.2021.154712 [DOI] [PubMed] [Google Scholar]
- 133.Goodrich JK, Davenport ER, Beaumont M, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19(5):731–743. doi: 10.1016/j.chom.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin H, He QY, Shi L, Sleeman M, Baker MS, Nice EC. Proteomics and the microbiome: pitfalls and potential. Expert Rev Proteomics. 2019;16(6):501–511. doi: 10.1080/14789450.2018.1523724 [DOI] [PubMed] [Google Scholar]
- 135.Peng W, Huang J, Yang J, et al. Integrated 16S rRNA sequencing, metagenomics, and metabolomics to characterize gut microbial composition, function, and fecal metabolic phenotype in non-obese type 2 diabetic Goto-Kakizaki rats. Front Microbiol. 2019;10:3141. doi: 10.3389/fmicb.2019.03141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou W, Sailani MR, Contrepois K, et al. Longitudinal multi-omics of host-microbe dynamics in prediabetes. Nature. 2019;569(7758):663–671. doi: 10.1038/s41586-019-1236-x [DOI] [PMC free article] [PubMed] [Google Scholar]