Summary
Background
The synergistic effect of locoregional therapy in combination with systemic therapy as a conversion therapy for unresectable hepatocellular carcinoma (uHCC) is unclear. The purpose of this study was to evaluate the efficacy and safety of transcatheter arterial chemoembolisation (TACE) combined with lenvatinib and camrelizumab (TACE + LEN + CAM) as conversion therapy for uHCC.
Methods
This single-arm, multicentre, prospective study was conducted at nine hospitals in China. Patients (aged 18–75 years) diagnosed with uHCC, an Eastern Cooperative Oncology Group performance score (ECOG-PS) of 0–1 and Child-Pugh class A received camrelizumab (200 mg, every 3 weeks) and lenvatinib (bodyweight ≥60 kg: 12 mg/day; <60 kg: 8 mg/day) after TACE treatment. Surgery was performed after tumour was assessed as meeting the criteria for resection. Patients who did not meet the criteria for surgery continued to receive triple therapy until disease progression or intolerable toxicity. Primary endpoints were objective response rate (ORR) according to the modified Response Evaluation Criteria in Solid Tumours (mRECIST) and safety. Secondary endpoints included the surgical conversion rate, radical (R0) resection rate, and disease control rate (DCR). This study was registered with Chinese Clinical Trial Registry (ChiCTR2100050410).
Findings
Between Oct 25, 2021, and July 20, 2022, 55 patients were enrolled. As of the data cutoff on June 1, 2023, the median follow-up was 13.3 months (IQR 10.6–15.9 months). The best tumour response to triple therapy was complete response (CR) in 9 (16.4%) patients, partial response (PR) in 33 (60.0%) patients, stable disease (SD) in 5 (9.1%) patients, or progressive disease (PD) in 7 (12.7%) patients. The ORR was 76.4% (42/55, 95% CI, 65.2–87.6%), and the DCR was 85.5% (47/55, 95% CI, 76.2–94.8%) per mRECIST. Twenty-four (43.6%) of the 55 patients suffered from grade 3–4 treatment-related adverse events (TRAEs). No grade 5 TRAEs occurred. A total of 30 (30/55, 54.5%) patients were converted to resectable HCC and 29 (29/55, 52.7%) patients underwent resection. The R0 resection rate was 96.6% (28/29). The major pathologic response (MPR) and pathologic complete response (pCR) rates in the surgery population were 65.5% (19/29) and 20.7% (6/29), respectively. Only one patient developed a Clavien-Dindo IIIa complication (abdominal infection). No Clavien-Dindo IIIb-V complications occurred. The median OS and median PFS were not reached.
Interpretation
The triple therapy (TACE + LEN + CAM) is promising active for uHCC with a manageable safety. Moreover, triple therapy has good conversion efficiency and the surgery after conversion therapy is feasible and safe. To elucidate whether patients with uHCC accepting surgical treatment after the triple therapy can achieve better survival benefits than those who receive triple therapy only, well-designed randomised controlled trials are needed.
Funding
This study was funded by the Natural Science Foundation of Fujian Province, China (2022J01691) and the Youth Foundation of Fujian Province Health Science and Technology Project, China (2022QNA035).
Keywords: Unresectable hepatocellular carcinoma (uHCC), Conversion therapy, Transcatheter arterial chemoembolisation (TACE), Lenvatinib, PD-1 inhibitor
Research in context.
Evidence before this study
Conversion therapy is one of the important approaches for patients with unresectable hepatocellular carcinoma (uHCC) to obtain radical treatment and long-term survival. Although the combination of transcatheter arterial chemoembolisation (TACE), tyrosine kinase inhibitor and immune checkpoint inhibitors is expected to be synergistic, their efficacy and safety have not been prospectively explored in patients with uHCC. We searched PubMed, with no language restrictions, for studies published from the inception of the database until August 22, 2023, using the search terms (“unresectable” AND “hepatocellular carcinoma” AND “TACE” AND “tyrosine kinase inhibitor” AND “immune checkpoint inhibitor”). We found retrospective studies and meta-analyses, but no prospective multicentre trials investigating the efficacy and safety of TACE combined with lenvatinib and camrelizumab (TACE + LEN + CAM) for uHCC. We aimed to address this gap.
Added value of this study
To our best knowledge, this is the first prospective multicentre study to evaluate the efficacy and safety of TACE combined with lenvatinib and camrelizumab for uHCC. Our study provides further evidence for the positive effects of triple therapy on objective response rate and safety in patients with uHCC. Moreover, triple therapy has astonishing conversion efficiency and the surgery after conversion therapy is feasible and safe.
Implications of all the available evidence
Our results suggest that triple therapy (TACE + LEN + CAM) is a promising conversion therapy for patients with uHCC. The encouraging results from our proposed treatment regimen provide a potential strategy for patients with uHCC to obtain radical treatment. Future work is warranted. In order to elucidate whether patients with uHCC accepting surgical treatment after the triple therapy can achieve better survival benefits than those who receive triple therapy only, well-designed randomised controlled trials are needed.
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide, and accounts for 4.7% of all new cancer cases and 8.3% of all cancer-related deaths, ranking sixth and third, respectively.1 Due to the insidious onset of HCC, 70–80% of patients are initially diagnosed at an intermediate or advanced stage and can only receive non-surgical treatment, while only 20–30% of patients have access to surgery.2, 3, 4 With the progress of research in recent years, more and more treatment modalities are being applied to HCC, but surgery is still the predominant treatment to obtain radical cure for patients with HCC.5,6
Transcatheter arterial chemoembolisation (TACE), as the first-line recommended treatment for intermediate and advanced HCC, can directly increase the local concentration of chemotherapeutic drugs in tumour tissues and block the blood supply to tumour cells, leading to necrosis and apoptosis of tumour cells.7,8 However, the tumour response rate of TACE monotherapy is limited. In patients with large or multinodular HCC, the tumour response rate was only about 30%.9,10 Over the past decade, systemic therapeutic agents (multikinase inhibitors and immune checkpoint inhibitors [ICIs]) for HCC have rapidly evolved.11 But unfortunately, the tumour response rates of monotherapy with these drugs were modest, ranging between 3.3% and 24.1%.12,13 Due to the synergistic effect, the combination of ICIs and antiangiogenic agents showed exciting results.14, 15, 16, 17 A recent study showed that pembrolizumab combined with lenvatinib for unresectable HCC (uHCC) achieved an objective response rate (ORR) of 46% and a median OS of 22 months.18 Recently, a randomised, international phase 3 trial (CARES-310) suggested that camrelizumab plus rivoceranib showed a statistically significant and clinically meaningful benefit in progression-free survival (PFS) and overall survival (OS) compared with sorafenib for patients with uHCC.19 These results suggested a promising therapeutic potential of the combination of multikinase inhibitors plus ICIs in patients with HCC. However, the use of either TACE or systemic therapy only modestly affected the ability to downstage locally advanced, unresectable HCC for curative surgery. To date, there is no established conversion treatment regimen.
Data suggested that locoregional therapy could modulate the tumour microenvironment, enhancing the tumour response to ICIs.20 Recently, several observational studies have shown that TACE combined with systemic therapy can significantly improve the tumour response and prognosis in intermediate and advanced HCC.21, 22, 23, 24 However, most of the studies are retrospective, and the use of PD-1 inhibitors or molecular targeted therapies was not uniform. Several randomised controlled trials (RCTs), such as LEAP-012 and EMERAD-3, have been designed to validate the clinical benefit of TACE with ICIs plus molecular targeted therapies in HCC. To date, these RCTs are still in patient recruitment stage and no relevant results have been published. Most of these studies examined the triple therapy as a first-line treatment option, rather than conversion therapy. Meanwhile, only few studies have reported successful conversion to surgery. Therefore, we conducted this single-arm, multicentre, prospective study to evaluate the efficacy and safety of the triple therapy (TACE + LEN + CAM) as conversion therapy in patients with uHCC.
Methods
Study design and patients
This prospective, single-arm study investigated the efficacy and safety of sequential TACE, lenvatinib, and camrelizumab in patients with uHCC, and was conducted at nine hospitals in China. The 9 hospitals we selected were all grade-A tertiary hospitals in China, qualified for clinical research and skilled in performing laparoscopic anatomic hepatectomy. We established a study management committee consisting of investigators from each centre, and all enrolled patients were evaluated and confirmed by the committee. Patients with successful conversion were also confirmed and surgical protocols discussed by the study management committee. We had an independent team to follow up with each enrolled patient and kept a detailed record of each treatment process, response and effect. In these ways, we ensured the homogeneity of this study as much as possible.
The inclusion criteria for this study were as follows: 1) aged between 18 and 75 years; 2) diagnosis of uHCC with at least one measurable lesion (computed tomography [CT] scan of the measurable lesion ≥10 mm in length or the malignant lymph node ≥15 mm in short diameter as per the modified Response Evaluation Criteria in Solid Tumours [mRECIST]25); 3) no previous treatment with systemic therapeutic agents; 4) China liver cancer staging (CNLC) IIa-IIIb; 5) Child-Pugh class A; 6) Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0–1. uHCC was defined as extensive bilobar involvement of the liver due to a large solitary or multiple tumours, or invasion of major vessels including the main trunk of portal vein (Vp4) and inferior vena cava (Vv3) according to the Liver Cancer Study Group of Japan.26
The exclusion criteria for this study were as follows: 1) combined with autoimmune disease, organ/hematopoietic stem cell transplantation or other malignancies; 2) prior treatment with TACE, molecular targeted therapies, camrelizumab or other PD-1/PD-L1 inhibitors; 3) undergoing major surgery, or treatment with chemotherapy, radiation therapy or other systemic treatment for the lesions within 1 month prior to enrolment; 4) immunosuppressive or systemic hormone therapy within 14 days prior to enrolment; 5) severe liver, kidney, heart, lung, brain, and other major organ failure; 6) other contraindications to interventional, molecular targeted therapy, and immunotherapy.
Ethics
The trial was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki, and applicable local regulations. The protocol was reviewed and approved by the Chinese Ethics Committee of Registering Clinical Trials (ChiECRCT20210409). All patients provided written informed consent before study enrolment. This study was registered with Chinese Clinical Trial Registry (ChiCTR2100050410).
Procedures
Patients were treated with conventional TACE within 3 days of enrolment. Conventional TACE was performed under local anaesthesia with selective hepatic angiography to identify the tumour-feeding vessels. Then an emulsion of lipiodol and lobaplatin was injected through the microcatheter into the tumour-feeding vessels, followed by embolisation with gelatine sponge particles until complete arterial flow stasis was observed. If contrast-enhanced CT or magnetic resonance imaging (MRI) revealed dense iodine oil deposition, obvious necrosis of the tumour, and no enlargement or new lesions, re-treatment with TACE was not considered. The frequency of TACE was determined by the follow-up results during the treatment period. Lenvatinib was discontinued for at least 3 days before and after TACE.
After 3 days of TACE, patients received lenvatinib (8 mg for bodyweight <60 kg or 12 mg for bodyweight ≥60 kg) orally once daily and camrelizumab (200 mg) intravenously every 3 weeks (plus or minus 3 days). Patients with successful conversion to resectable HCC received surgery by the principal investigator of each centre after multidisciplinary discussion and evaluation. Lenvatinib was discontinued for 3 weeks and surgical resection was performed within 2–4 weeks of the last camrelizumab treatment. For patients undergoing surgery, it was at the patient's discretion to receive adjuvant therapy. Adjuvant therapy with camrelizumab and lenvatinib was recommended within 4 weeks after surgery and continued for at least 6 months. Efficacy evaluation was performed every 3 months during adjuvant therapy. If there was no recurrence or metastasis of HCC on two consecutive efficacy evaluations, the adjuvant therapy was considered to be discontinued. Patient who did not meet the criteria for surgery continued to receive the triple therapy until progressive disease, intolerable toxicity, refusal of treatment, and other conditions that the investigators considered necessary to discontinue treatment.
All patients with active HBV infection received oral antiviral therapy (entecavir). Patients with HBV-DNA ≥2000IU/mL received antiviral therapy at least 1 week before triple therapy. Before the treatment, all patient underwent a detailed history taking, physical examination, laboratory examinations (including blood routine examination, coagulation function, liver, kidney, thyroid, cardiac function, hepatitis B surface antigen [HBsAg], HBV-DNA, serum α-fetoprotein [AFP], protein induced by vitamin K absence-II [PIVKA-II]) and baseline radiological examinations (CT of the thorax, contrast-enhanced CT and/or MRI of the liver). During treatment, follow-up was conducted every 3 weeks, including a history taking, physical examination and laboratory tests. Radiological examinations (CT/MRI) were performed every 6–8 weeks (±7 days). According to mRECIST, the tumour response was assessed as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Changes in AFP and PIVKA-II levels of patients were also recorded. After discontinuation of treatment, all patients were followed up for survival every 3 months in the first year and every 6 months thereafter.
Treatment-related adverse events (TRAEs) were recorded throughout the treatment period and assessed based on National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (NCI-CTCAE, v5.0).
Outcomes
The primary endpoints were ORR and TRAEs. The secondary endpoints included the surgical conversion rate, radical (R0) resection rate, and disease control rate (DCR). ORR was defined as the percentage of patients with the best response (either CR or PR) ≥4 weeks. DCR was defined as the percentage of patients with CR, PR, and SD. Time to response (TTR) was defined as the time from treatment initiation to achievement of PR or CR. The criteria of resectable HCC were defined as follows: 1) R0 resection with preservation of a sufficient remnant liver volume could be achieved; 2) Child-Pugh class A or B; 3) ECOG-PS score 0–1; 4) no tumour thrombus in the main trunk of portal vein and inferior vena cava; and 5) no contraindications for hepatectomy. Pathologic complete response (pCR) and major pathologic response (MPR) were defined as the complete absence and ≤10% of viable tumour cells in the resected specimen, respectively.
Statistical analysis
According to previous studies, the ORR of camrelizumab combined with apatinib as the first line treatment in advanced HCC is 34.3% and the ORR of pembrolizumab combined with lenvatinib is 46.0% (H0 = 46%) per mRECIST in uHCC.18,27 Although we do not conduct any formal statistical test, at study design we expected that the regimen of TACE combined with lenvatinib plus camrelizumab could increase the ORR from 46% to 66% (Hl = 66%), and a sample size of 49 patients would provide at least 80% power to detect this estimated improvement at a two-sided a level of 5%. Considering an approximate drop-out incidence of 10%, a total sample size of 54 patients was planned for this study.
The full analysis set (FAS) and safety analysis set (SAS), both of which comprised all eligible patients who received at least one dose of lenvatinib plus camrelizumab and once TACE, were used to conduct the efficacy and safety assessment, respectively. A post-hoc blinded analysis of the tumour responses per RECIST v1.1 was performed by two radiologists. A third blinded, experienced radiologist reviewed the results when there was uncertainty or disagreement and gave the final result.
Continuous data were summarised as mean ± standard deviation and median (interquartile range [IQR]) for normally and nonnormally distributed variables, respectively. Categorical data were summarised as number (percentage). The percentages and 95% confidence intervals for ORR, and DCR were estimated using the Clopper-Pearson method.
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software (Version 24, SPSS, Inc., Chicago, IL, USA). The protocol is available in the Suppl Materials.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
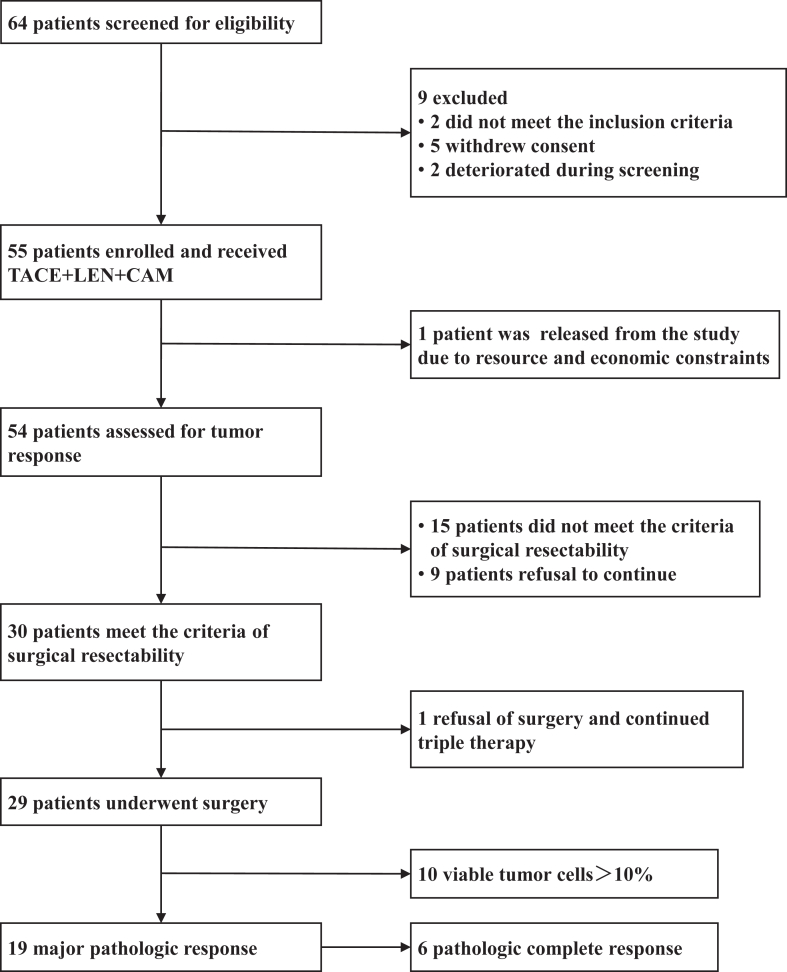
Between October 2021 and July 2022, 55 patients from nine hospitals in China were enrolled and received the triple therapy, and were included in the FAS and SAS (Fig. 1). Of the 55 patients included, one patient was released from the study due to resource and economic constraints and 54 patients had undergone efficacy assessment; 9 patients refused further treatment; and 15 patients did not meet the criteria for surgery and continued conversion treatment. Thirty (30/55, 54.5%) patients met the criteria for surgery, of which one patient refused surgery and continued to receive the triple therapy. A total of 29 (29/55, 52.7%) patients underwent hepatectomy.
Fig. 1.
Flow diagram of patient enrolment.
The baseline and disease characteristics of the enrolled patients are shown in Table 1. Of the 55 patients, the median age was 54 years (IQR, 46–62 years), and 45 (81.8%) patients were male. More than half of the patients had CNLC stage IIIa disease (32/55, 58.2%). A total of 12 (21.8%) patients were categorised as Barcelona Clinic Liver Cancer (BCLC) stage B and 43 (78.2%) were BCLC stage C. Twenty-seven (49.1%) patients had hepatitis B virus infection. Thirty-two (32/55, 58.2%) patients had baseline AFP ≥400 ng/mL, and 47 (85.5%) patients had baseline PIVKA-II ≥400 mAU/mL. Thirty-seven (67.3%) patients had portal vein tumour thrombosis (PVTT). The mean of maximum tumour diameter was 101.7 ± 42.3 mm, and 23 (41.8%) patients had multiple tumours. Ten (18.2%) patients had extrahepatic metastases, and the most common metastasis site was lung. The median duration of lenvatinib treatment was 2.6 months (IQR, 1.6–3.3 months). The median cycle of camrelizumab treatment was 3 (IQR, 2–4). The median number of TACE treatments was 1 (IQR, 1–2).
Table 1.
Patient demographics and baseline characteristics.
| Patients (n = 55) | |
|---|---|
| Age (years), median (IQR) | 54 (46–62) |
| Sex, n (%) | |
| Male | 45 (81.8%) |
| Female | 10 (18.2%) |
| ECOG-PS, n (%) | |
| 0 | 47 (85.5%) |
| 1 | 8 (14.5%) |
| Child-Pugh, n (%) | |
| 5 | 24 (43.6%) |
| 6 | 31 (56.4%) |
| Hepatitis B infection, n (%) | |
| Yes | 27 (49.1%) |
| No | 28 (50.9%) |
| HBV-DNA copy, n (%) | |
| <2000 copy/mL | 31 (56.4%) |
| ≥2000 copy/mL | 19 (34.5%) |
| Unknown | 5 (9.1%) |
| AFP, n (%) | |
| <400 ng/mL | 23 (41.8%) |
| ≥400 ng/mL | 32 (58.2%) |
| PIVKA-II, n (%) | |
| <400 mAU/mL | 7 (12.7%) |
| ≥400 mAU/mL | 47 (85.5%) |
| Unknown | 1 (1.8%) |
| PVTT type, n (%) | 37 (67.3%) |
| I | 4 (7.3%) |
| II | 26 (47.3%) |
| III | 4 (7.3%) |
| IV | 3 (5.5%) |
| Extrahepatic metastasis, n (%) | |
| Yes | 10 (18.2%) |
| No | 45 (81.8%) |
| Extrahepatic metastasis location, n (%) | |
| Lung | 5 (9.1%) |
| Lymph node | 4 (7.3%) |
| Adrenal gland | 1 (1.8%) |
| Tumour number, n (%) | |
| Solitary | 32 (58.2%) |
| Multiple | 23 (41.8%) |
| Maximum tumour size (mm), mean ± SD | 101.7 ± 42.3 |
| CNLC staging, n (%) | |
| Ⅱa | 4 (7.3%) |
| Ⅱb | 9 (16.4%) |
| Ⅲa | 32 (58.2%) |
| Ⅲb | 10 (18.2%) |
| BCLC staging, n (%) | |
| B | 12 (21.8%) |
| C | 43 (78.2%) |
| Duration of lenvatinib (months), median (IQR) | 2.6 (1.6–3.3) |
| Cycles of camrelizumab, median (IQR) | 3 (2–4) |
| Number of TACE, median (IQR) | 1 (1–2) |
ECOG-PS, Eastern Cooperative Oncology Group performance status; AFP, α-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; PVTT, portal vein tumour thrombosis; CNLC, China liver cancer staging; BCLC, Barcelona Clinic liver cancer stage; TACE, transcatheter arterial chemoembolisation.
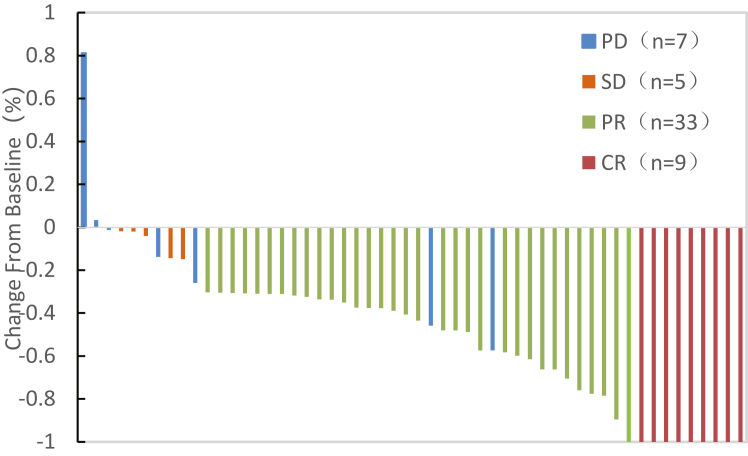
As of data cutoff on June 1, 2023, the median follow-up was 13.3 months (IQR 10.6–15.9 months). 54 patients had completed the tumour response evaluation and 1 patient was dropped out before evaluation. The tumour response of the eligible patients is shown in Table 2. According to mRECIST, 9 (16.4%) patients achieved a confirmed CR, 33 (60.0%) patients achieved a confirmed PR, 5 (9.1%) patients had SD and 7 (12.7%) patients experienced PD. The ORR of this study was 76.4% (42/55, 95% CI, 65.2–87.6%) and the DCR was 85.5% (47/55, 95% CI, 76.2–94.8%) per mRECIST. Waterfall analysis showed viable tumour component reduction in 94.5% (52/55) of the patients per mRECIST (Fig. 2). The ORR of this study was 20.0% (11/55, 95% CI, 9.4–30.6%) and the DCR was 85.5% (47/55, 95% CI, 76.2–94.8%) per RECIST v1.1 (Supplementary Table S1). The median TTR was 2.1 months (IQR, 1.5–3.3 months). At data cutoff, 19 of the 55 patients had disease progression and 4 patients had died. The median OS and median PFS was not reached.
Table 2.
Best tumour responses as per mRECIST.
| Patients (n = 55) | |
|---|---|
| Complete response, n (%) | 9 (16.4%) |
| Partial response, n (%) | 33 (60.0%) |
| Stable disease, n (%) | 5 (9.1%) |
| Progressive disease, n (%) | 7 (12.7%) |
| Not evaluable, n (%) | 1 (1.8%) |
| Objective response rate, n (%, 95% CI) | 42 (76.4%, 65.2–87.6%) |
| Disease control rate, n (%, 95% CI) | 47 (85.5%, 76.2–94.8%) |
Fig. 2.
Waterfall plot of the best percent change in viable target lesion per mRECIST.
All the 55 (100%) patients reported TRAEs of any grade and 24 (43.6%) patients experienced grade 3–4 TRAEs. Among these, the most common TRAEs of any grade were aspartate aminotransferase (AST) increased (36/55, 65.5%), alanine aminotransferase (ALT) increased (34/55, 61.8%), pyrexia (25/55, 45.5%), lymphopenia (22/55, 40.0%), and electrolytes disorder (21/55, 38.2%) (Table 3). Four of the 55 patients developed grade 4 TRAEs, including one with AST and ALT increased and three with anaphylaxis. No grade 5 TRAEs occurred. Six (10.9%) patients experienced adverse events leading to dose modifications or interruptions. Three patients permanently discontinued camrelizumab because of anaphylactic shock. One patient discontinued camrelizumab permanently and another patient discontinued camrelizumab once because of infusion-related reactions. One patient discontinued lenvatinib permanently because of the development of hepatic encephalopathy (TRAEs of special interest was listed in Supplementary Table S2).
Table 3.
Treatment-related adverse events occurring in >10% of patients.
| Patients (n = 55) |
|||
|---|---|---|---|
| Any grade, n (%) | Grade 1–2, n (%) | Grade 3–4, n (%) | |
| Any adverse event | 55 (100%) | 31 (56.3%) | 24 (43.6%) |
| Elevated AST | 36 (65.5%) | 25 (45.5%) | 11 (20.0%) |
| Elevated ALT | 34 (61.8%) | 27 (49.1%) | 7 (12.7%) |
| Pyrexia | 25 (45.5%) | 21 (38.2%) | 4 (7.3%) |
| Lymphopenia | 22 (40.0%) | 18 (32.7%) | 4 (7.3%) |
| Electrolytes disorder | 21 (38.2%) | 20 (36.4%) | 1 (1.8%) |
| Hypoalbuminemia | 21 (38.2%) | 21 (38.2%) | 0 |
| Anaemia | 20 (36.4%) | 18 (32.7%) | 2 (3.6%) |
| Thrombocytopenia | 20 (36.4%) | 18 (36.3%) | 2 (3.6%) |
| Elevated GGT | 19 (34.5%) | 18 (32.7%) | 1 (1.8%) |
| Fatigue | 18 (32.7%) | 18 (32.7%) | 0 |
| Nausea | 18 (32.7%) | 18 (32.7%) | 0 |
| Abdominal pain | 15 (27.3%) | 15 (27.3%) | 0 |
| Hypothyroidism | 15 (27.3%) | 15 (27.3%) | 0 |
| Leucocytosis | 15 (27.3%) | 15 (27.3%) | 0 |
| Vomiting | 14 (25.5%) | 14 (25.5%) | 0 |
| Elevated ALP | 14 (25.5%) | 14 (25.5%) | 0 |
| Leukocytopenia | 13 (23.6%) | 13 (23.6%) | 0 |
| Hyperbilirubinemia | 12 (21.8%) | 11 (20.0%) | 1 (1.8%) |
| Elevated PT | 11 (20.0%) | 11 (20.0%) | 0 |
| Shiver | 9 (16.4%) | 9 (16.4%) | 0 |
| Cough | 7 (12.7%) | 7 (12.7%) | 0 |
| RCCEP | 7 (12.7%) | 7 (12.7%) | 0 |
| Stress hyperglycaemia | 7 (12.7%) | 7 (12.7%) | 0 |
| Hypertension | 6 (10.9%) | 6 (10.9%) | 0 |
| Arrhythmias | 6 (10.9%) | 6 (10.9%) | 0 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; PT, prothrombin time; RCCEP, reactive cutaneous capillary endothelial proliferation.
Among the 29 patients who underwent surgical resection, the median number of TACE treatment was 1 (IQR, 1–2), and the median cycles of treatment with camrelizumab and lenvatinib was 3 (IQR, 2–4). The median time from initiation of the triple therapy to surgical resection was 2.8 months (IQR, 1.8–3.4 months). The mean operative time was 3.7 ± 1.1 h, and the median intraoperative blood loss was 300 mL (IQR, 200–400 mL). The R0 resection rate was 96.6% (28/29). The pathological results after the operation showed that there were 19 (19/29, 65.5%) patients with MPR, including 6 (6/29, 20.7%) patients with pCR. The median postoperative hospital stays of those patients was 10 days (IQR, 8.5–13 days). Only one patient experienced a Clavien-Dindo IIIa complication (abdominal infection). No Clavien-Dindo IIIb-V complications occurred (Table 4).
Table 4.
Perioperative outcomes.
| Patients (n = 29) | |
|---|---|
| Surgical conversion rate, n/N (%) | 29/55 (52.7%) |
| Cycles of CAM + LEN, median (IQR) | 3 (2–4) |
| Number of TACE, median (IQR) | 1 (1–2) |
| Time from the start of triple therapy to surgical resection (months), median (IQR) | 2.8 (1.8,3.4) |
| Surgical type, n (%) | |
| Laparoscopic | 6 (20.7%) |
| Open | 23 (79.3%) |
| Operative time (h), mean ± SD | 3.7 ± 1.1 |
| Blood loss (mL), median (IQR) | 300 (200, 400) |
| Difficulty of operation, n (%) | |
| Increased | 0 |
| Not increased | 29 (100%) |
| R0 resection rate, n (%) | 28 (96.6%) |
| R1resection rate, n (%) | 1 (3.4%) |
| pCR, n (%) | 6 (20.7%) |
| MPR, n (%) | 19 (65.5%) |
| Clavien-Dindo classification, n (%) | |
| 0-II | 28 (96.6%) |
| III-V | 1 (3.4%) |
| Postoperative hospital stays (days), median (IQR) | 10 (8.5, 13) |
CAM, camrelizumab; LEN, lenvatinib; TACE, transcatheter arterial chemoembolisation; pCR, pathologic complete response; MPR, major pathologic response.
There were 27 (93.1%) patients with surgical resection received adjuvant therapy. Two patients refused postoperative adjuvant therapy for economic reasons. There were 20 (69.0%) patients continued to receive camrelizumab and lenvatinib, of which 6 patients received TACE once to prevent tumour recurrence. Six patients received only lenvatinib or camrelizumab as adjuvant therapy due to resource and economic constraints. One patient was switched to penpulimab as adjuvant therapy because of recurrence within 4 weeks after surgery. The median cycles of postoperative adjuvant therapy for all the 27 patients were 2 (IQR, 1–4). With a median postoperative follow-up of 10.3 months (IQR, 9.1–13.2 months), only 4 (11.5%) patients had postoperative tumour recurrence (three intrahepatic and one pulmonary), with the relapse-free survival of 3.3, 3.4, 3.8, and 6.3 months, respectively (Table 5).
Table 5.
Postoperative recurrence and adjuvant therapy.
| Patients (n = 29) | |
|---|---|
| Time of postoperative follow-up (months), median (IQR) | 10.3 (9.1–13.2) |
| Number of patients received adjuvant therapy, n (%) | 27 (93.1%) |
| LEN + CAM | 14 (48.3%) |
| TACE + LEN + CAM | 6 (20.7%) |
| CAM | 3 (10.3%) |
| TACE + CAM | 2 (6.9%) |
| TACE + LEN | 1 (3.4%) |
| TACE + Penpulimab | 1 (3.4%) |
| Adjuvant therapy cycles, median (IQR) | 2 (1–4) |
| Number of patients with HCC recurrence, n (%) | 4 (13.8%) |
| Intrahepatic | 3 (10.3%) |
| Pulmonary | 1 (3.4%) |
| Time of recurrence (months) | 3.3, 3.4, 3.8, and 6.3 |
CAM, camrelizumab; LEN, lenvatinib; TACE, transcatheter arterial chemoembolisation; HCC, hepatocellular carcinoma.
Discussion
Despite the growing number of treatments, the prognosis of HCC is poor, especially for patients with advanced, unresectable HCC with a median survival of less than 1 year.5 Fortunately, the results of some retrospective studies have shown higher survival rates after conversion resection compared to palliative treatment.28, 29, 30, 31 However, such retrospective studies may be subject to selection bias and there is no standardisation of the definition of uHCC or the criteria for surgical resection, thus affecting the comparability of survival data. In this trial, TACE combined with lenvatinib and camrelizumab in patients with uHCC resulted in 54.5% of patients becoming amenable to curative treatment, and 52.7% of patients undergoing curative treatment. To the best of our knowledge, this study was the first prospective clinical trial using TACE combined with lenvatinib and camrelizumab as conversion therapy for uHCC. Moreover, this triple therapy achieved a promising ORR of 76.4%, a DCR of 85.5% as per mRECIST, and a median TTR of only 2.1 months. Overall, our study suggested that TACE combined with lenvatinib and camrelizumab was effective with a rapid treatment response and promising conversion rate.
Previous studies have assessed the combination of TACE, lenvatinib and PD-1 inhibitor for patients with uHCC and reported an ORR of 46.7%–72.7%.23,24,32, 33, 34 However, these studies were retrospective and various kinds of PD-(L)1 inhibitors or molecular targeted therapies were used, which may lead to difficulties in identifying the effects of specific combination regimens. Different PD-(L)1 inhibitors or molecular targeted therapies may have different combination effects on HCC. CHANCE2211 is a propensity score-matched study with a specific combination regimen of TACE plus camrelizumab and apatinib, achieved an ORR of 59.5%.35 However, none of these studies used combination regimen as a conversion therapy for uHCC. In our prospective study, we focused on the surgical conversion rate and perioperative safety of TACE combined with lenvatinib and camrelizumab as a conversion therapy. The results suggested that TACE combined with lenvatinib and camrelizumab had astonishing conversion efficiency and the surgery after conversion was feasible and safe.
Although several clinical studies have confirmed that TACE combined with lenvatinib and PD-1 inhibitor was a better treatment option for advanced HCC, the exact mechanism is still unclear. The effectiveness of triple therapy may be attributed to the following reasons: 1) TACE is able to reduce the blood supply to the tumour, leading to extensive local necrosis of tumour cells and the release of large amounts of tumour-specific antigens, which would enhance the antitumour immune effect of PD-1 inhibitors36; 2) After TACE, the hypoxic micro-environment can lead to tumour angiogenesis.37 Lenvatinib is a multikinase inhibitor, which may counteract post-TACE angiogenesis by targeting VEGF 1–3, FGFR 1–4, PDGFRα, KIT, and RET38,39; 3) Lenvatinib modulates cancer immunity in the tumour microenvironment by reducing tumour-associated macrophages (TAMs).40 When combined with PD-1 blockade, it shows enhanced antitumour activity via the IFN signalling pathway.41 In this study, the combination of TACE, lenvatinib, and camrelizumab demonstrated a synergistic antitumour effect, leading to better clinical outcomes in patients with uHCC. But the exact mechanism of interaction needs further investigation.
The need for surgery in patients who have achieved imaging remission after treatment is inconclusive. Even with continued treatment, most patients in remission experience disease progression after 1–1.5 years.14,18 There is no evidence to support that HCC patients with radiological complete remission can achieve long-term survival with continued non-surgical treatment. Surgical treatment for patients with successful conversion can not only reduce tumour load but also reduce TRAEs associated with ongoing systemic therapy. Notably, in our study, 5 patients with preoperative radiological CR had MPR, while 2 patients with radiological PR had pCR. It means that postoperative pathology can accurately determine the degree of tumour remission and guide the next adjuvant therapy. In this study, a total of 29 patients underwent surgery, with the surgical conversion rate of 52.7%, the MPR rate of 65.5% and pCR rate of 20.7%. Postoperative complications of Clavien-Dindo III occurred in only one patient. In general, triple therapy regimen (TACE + LEN + CAM) had a high conversion rate, and the post-conversion surgery was effective with mild complications. Therefore, we believe that patients who meet surgical resection criteria after conversion therapy should receive curative surgery as much as possible, but further data are needed to demonstrate the prognosis of patients undergoing post-conversion surgery.
The safety profile of the triple therapy regimen was consistent with the safety profile previously observed with each individual treatment.13,38,42 All TRAEs were manageable and there were no new or unexpected TRAEs observed. Among all patients, only 4 patients developed grade 4 TRAEs and no patient developed grade 5 TRAEs. Only 6 patients had treatment dose adjustments or interruption. For patients with successful conversion, the surgery was feasible with manageable postoperative complications.
The present study has some limitations. First, although this study was conducted at nine hospitals in China, the sample size is still limited and further expansion of the sample size is needed to increase the persuasiveness of this study. Second, the follow-up period of this study was short, and neither median OS nor median PFS has been achieved yet. Based on the current results of the available studies, it is indeed difficult to conclude that patients with advanced HCC can achieve a better survival benefit from conversion surgery than those who received triple therapy. The follow-up time of this study was short, and the survival data was incomplete and unstable. When the follow-up is complete, we will compare the survival of patients with and without conversion surgery and expect to clarify this issue at that time. Third, this study is a prospective single-arm study with no control group, which may induce some selection bias and could not clarify whether the triple therapy was superior to other treatment options. Therefore, well-designed RCTs are needed to further demonstrate the benefit of the triple therapy in patients with uHCC.
In conclusion, TACE combined with lenvatinib and camrelizumab exhibits promising ORR in patients with uHCC, with a manageable safety. Furthermore, this triple therapy has a promising conversion rate and offers the possibility for patients with uHCC to undergo curative surgical treatment. However, the long-term prognosis needs to be proven by further survival data.
Contributors
ZB Z, ML Y and YF C were responsible for trial conception, design and protocol written. ZW C, H L, XY S, MH C, L W, H Z, JF C, JY H, YY Z, and ML Y recruited patients and collected data. XK W and LF Y did the statistical analysis and evaluated images. XK W, LF Y and ZB Z accessed and verified all study data. XK W wrote the initial manuscript and ZB Z substantively revised it. All authors critically reviewed drafts of the manuscript and approved the final manuscript. All authors verified that this study was done according to the protocol and was attested for data accuracy and completeness. All authors had full access to all of the data in the study and accepted responsibility for the decision to submit the final manuscript for publication.
Data sharing statement
Individual patient data will not be available. The study protocol is available from the corresponding author on reasonable request.
Declaration of interests
We declare no competing interests.
Acknowledgements
All the co-authors would like to thank the patients and their families, the study investigators, coordinators, and research staff. Camrelizumab was provided by Jiangsu Hengrui Pharmaceuticals Co., Ltd. The post-hoc RECIST v1.1 analysis was supported by Hui-Hong Sun, Tan-Hui Chen, and De-Hua Chen, radiologists from the First Affiliated Hospital of Fujian Medical University.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102367.
Contributor Information
Mao-Lin Yan, Email: yanmaolin74@163.com.
Zhi-Bo Zhang, Email: zbzhang_1234@163.com.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.EASL Clinical Practice Guidelines Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 4.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 5.Reig M., Forner A., Rimola J., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J., Sun H., Wang Z., et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition) Liver Cancer. 2020;9(6):682–720. doi: 10.1159/000509424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Yu W., Zhang K., Liu W. Comparison of the efficacy and safety of Transarterial chemoembolisation with and without Apatinib for the treatment of BCLC stage C hepatocellular carcinoma. BMC Cancer. 2018;18(1):1131. doi: 10.1186/s12885-018-5081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C., Xing W., Si T., Yu H., Guo Z. Efficacy and safety of apatinib combined with transarterial chemoembolisation for hepatocellular carcinoma with portal venous tumour thrombus: a retrospective study. Oncotarget. 2017;8(59):100734–100745. doi: 10.18632/oncotarget.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shim S.J., Seong J., Han K.H., Chon C.Y., Suh C.O., Lee J.T. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolisation in locally advanced hepatocellular carcinoma. Liver Int. 2005;25(6):1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M., Han K.H., Ye S.L., et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–260. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo X.Y., Wu K.M., He X.X. Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets. J Exp Clin Cancer Res. 2021;40(1):172. doi: 10.1186/s13046-021-01968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M., Finn R.S., Qin S., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 14.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 15.Ren Z., Xu J., Bai Y., et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 16.El-Khoueiry A.B., Sangro B., Yau T., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu A.X., Finn R.S., Edeline J., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 18.Finn R.S., Ikeda M., Zhu A.X., et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin S., Chan S.L., Gu S., et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–1146. doi: 10.1016/S0140-6736(23)00961-3. [DOI] [PubMed] [Google Scholar]
- 20.Greten T.F., Mauda-Havakuk M., Heinrich B., Korangy F., Wood B.J. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70(5):999–1007. doi: 10.1016/j.jhep.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F., Xu G.L., Huang J.T., et al. Transarterial chemoembolisation combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: efficacy and systemic immune response. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.847601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke Q., Xin F., Fang H., Zeng Y., Wang L., Liu J. The significance of transarterial chemo(Embolisation) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: a systematic review. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.913464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai M., Huang W., Huang J., et al. Transarterial chemoembolisation combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.848387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J.Y., Yin Z.Y., Bai Y.N., et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolisation for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi: 10.2147/JHC.S332420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 26.Kudo M., Izumi N., Kokudo N., et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 27.Xu J., Shen J., Gu S., et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomised, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Huang G., Wang Y., et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolisation? Ten years of experience. Oncologist. 2016;21(12):1442–1449. doi: 10.1634/theoncologist.2016-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewandowski R.J., Kulik L.M., Riaz A., et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolisation versus radioembolisation. Am J Transplant. 2009;9(8):1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.S., Choi G.H., Choi J.S., et al. Surgical resection after down-staging of locally advanced hepatocellular carcinoma by localized concurrent chemoradiotherapy. Ann Surg Oncol. 2014;21(11):3646–3653. doi: 10.1245/s10434-014-3652-3. [DOI] [PubMed] [Google Scholar]
- 31.Kamiyama T., Nakanishi K., Yokoo H., et al. Efficacy of preoperative radiotherapy to portal vein tumour thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12(5):363–368. doi: 10.1007/s10147-007-0701-y. [DOI] [PubMed] [Google Scholar]
- 32.Cao F., Yang Y., Si T., et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.783480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H.D., Li H.L., Huang M.S., et al. Transarterial chemoembolisation with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001) Signal Transduct Targeted Ther. 2023;8(1):58. doi: 10.1038/s41392-022-01235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin Y., Zhang X., Liu N., et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolisation in unresectable hepatocellular carcinoma. Hepatol Int. 2023;17(3):753–764. doi: 10.1007/s12072-023-10502-3. [DOI] [PubMed] [Google Scholar]
- 35.Jin Z.C., Zhong B.Y., Chen J.J., et al. Real-world efficacy and safety of TACE plus camrelizumab and apatinib in patients with HCC (CHANCE2211): a propensity score matching study. Eur Radiol. 2023;33(12):8669–8681. doi: 10.1007/s00330-023-09754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheu J.W., Wong C.C. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74(4):2264–2276. doi: 10.1002/hep.31840. [DOI] [PubMed] [Google Scholar]
- 37.Kishore S.A., Bajwa R., Madoff D.C. Embolotherapeutic strategies for hepatocellular carcinoma: 2020 update. Cancers. 2020;12(4):791. doi: 10.3390/cancers12040791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang Y., Jeong S.W., Young Jang J., Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21):8165. doi: 10.3390/ijms21218165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumour burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8(5):299–311. doi: 10.1159/000502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato Y., Tabata K., Kimura T., et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumour-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adachi Y., Kamiyama H., Ichikawa K., et al. Inhibition of FGFR reactivates IFNγ signaling in tumour cells to enhance the combined antitumour activity of lenvatinib with anti-PD-1 antibodies. Cancer Res. 2022;82(2):292–306. doi: 10.1158/0008-5472.CAN-20-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangro B., Chan S.L., Meyer T., Reig M., El-Khoueiry A., Galle P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72(2):320–341. doi: 10.1016/j.jhep.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.