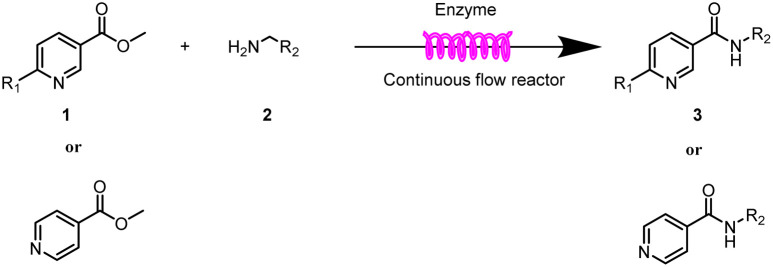
The effect of substrate structure to the enzymatic synthesis of coumarin carboxamide derivatives under continuous-flow conditionsa.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Methyl nicotinate | R 2 | Method | Time | Product | Yieldb (%) |
| 1 |
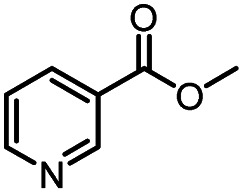
|
CH(CH3)2 | A | 35 min | 3a | 86.2 ± 1.5 |
| B | 24 h | 67.1 ± 0.8 | ||||
| 2 |
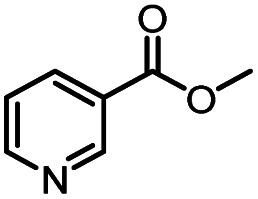
|
|
A | 35 min | 3b | 68.5 ± 0.8 |
| B | 24 h | 54.2 ± 0.9 | ||||
| 3 |
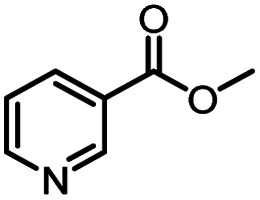
|
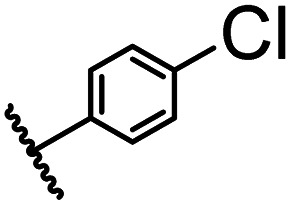
|
A | 35 min | 3c | 65.2 ± 1.5 |
| B | 24 h | 54.3 ± 1.2 | ||||
| 4 |
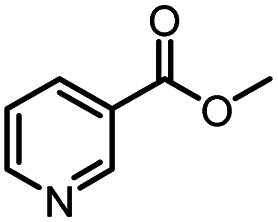
|
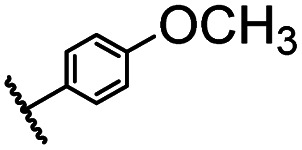
|
A | 35 min | 3d | 71.2 ± 0.8 |
| B | 24 h | 62.8 ± 0.9 | ||||
| 5 |
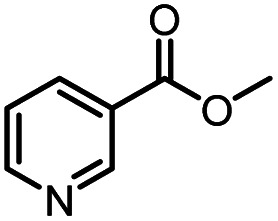
|
H | A | 35 min | 3e | 86.8 ± 0.4 |
| B | 24 h | 68.7 ± 0.9 | ||||
| 6 |
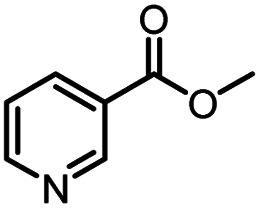
|
CH3 | A | 35 min | 3f | 83.3 ± 0.9 |
| B | 24 h | 75.9 ± 1.2 | ||||
| 7 |
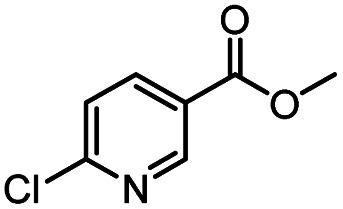
|
CH(CH3)2 | A | 35 min | 3g | 88.5 ± 0.7 |
| B | 24 h | 77.4 ± 1.5 | ||||
| 8 |
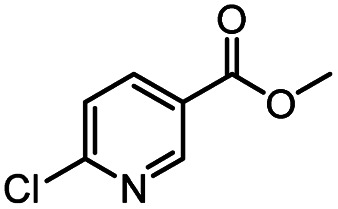
|
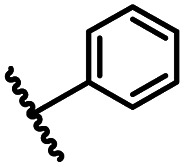
|
A | 35 min | 3h | 69.4 ± 1.8 |
| B | 24 h | 58.5 ± 0.7 | ||||
| 9 |
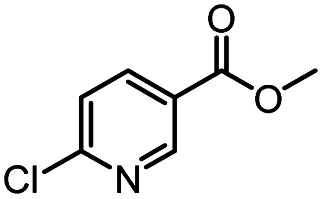
|
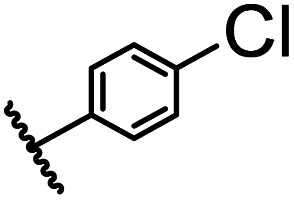
|
A | 35 min | 3i | 65.4 ± 0.5 |
| B | 24 h | 55.9 ± 0.8 | ||||
| 10 |
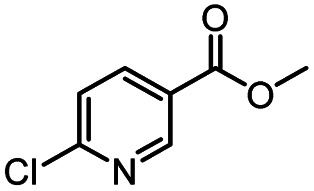
|
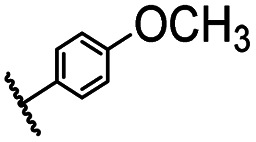
|
A | 35 min | 3j | 72.5 ± 0.7 |
| B | 24 h | 61.5 ± 0.5 | ||||
| 11 |
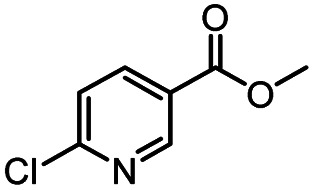
|
H | A | 35 min | 3k | 81.6 ± 0.7 |
| B | 24 h | 67.5 ± 0.7 | ||||
| 12 |
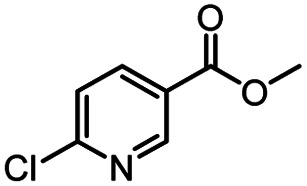
|
CH3 | A | 35 min | 3l | 85.6 ± 1.5 |
| B | 24 h | 65.9 ± 0.7 | ||||
| 13 |
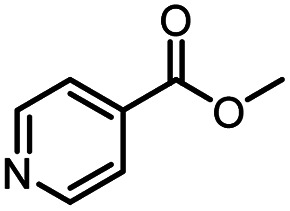
|
CH(CH3)2 | A | 35 min | 3m | 85.1 ± 1.2 |
| B | 24 h | 69.8 ± 0.7 | ||||
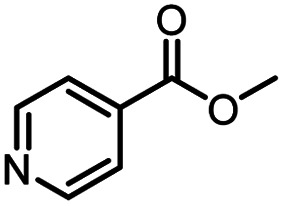
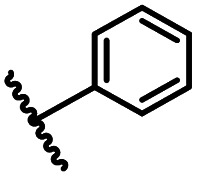
| 14 |
|
|
A | 35 min | 3n | 75.5 ± 0.7 |
| B | 24 h | 57.8 ± 0.5 | ||||
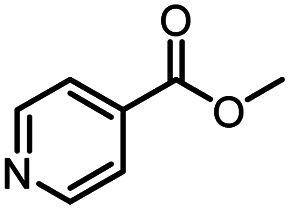
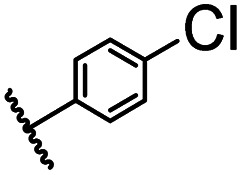
| 15 |
|
|
A | 35 min | 3o | 73.8 ± 0.9 |
| B | 24 h | 52.5 ± 0.6 | ||||
| 16 |
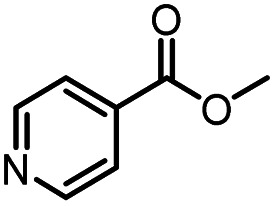
|
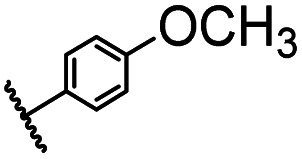
|
A | 35 min | 3p | 75.4 ± 1.5 |
| B | 24 h | 58.6 ± 0.8 | ||||
| 17 |
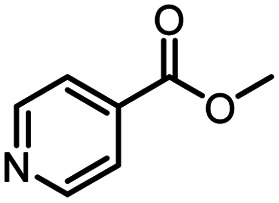
|
H | A | 35 min | 3q | 81.6 ± 1.2 |
| B | 24 h | 66.5 ± 0.7 | ||||
| 18 |
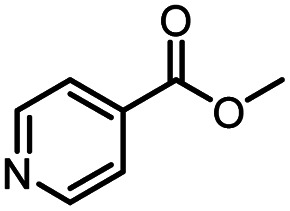
|
CH3 | A | 35 min | 3r | 82.2 ± 0.7 |
| B | 24 h | 62.3 ± 0.6 | ||||
General experimental conditions: Method A: continuous flow reactors, feed 1, dissolve 5 mmol of methyl nicotinate derivatives in 10 mL tert-amyl alcohol; feed 2, dissolve 10 mmol of amines in 10 mL tert-amyl alcohol, flow rate 17.8 μL min−1, residence time 35 min, enzyme 870 mg, 50 °C. Method B: shaker reactors, add 5 mmol of methyl nicotinate derivatives, 10 mmol of amines and 20 mL tert-amyl alcohol to a 50 mL Erlenmeyer flask, enzyme 870 mg, 160 rpm, 50 °C, 24 h.
Isolated yield. Yield: 100 × (actually obtained amount/calculated amount). The data are presented as average ± SD of triplicate experiments.
