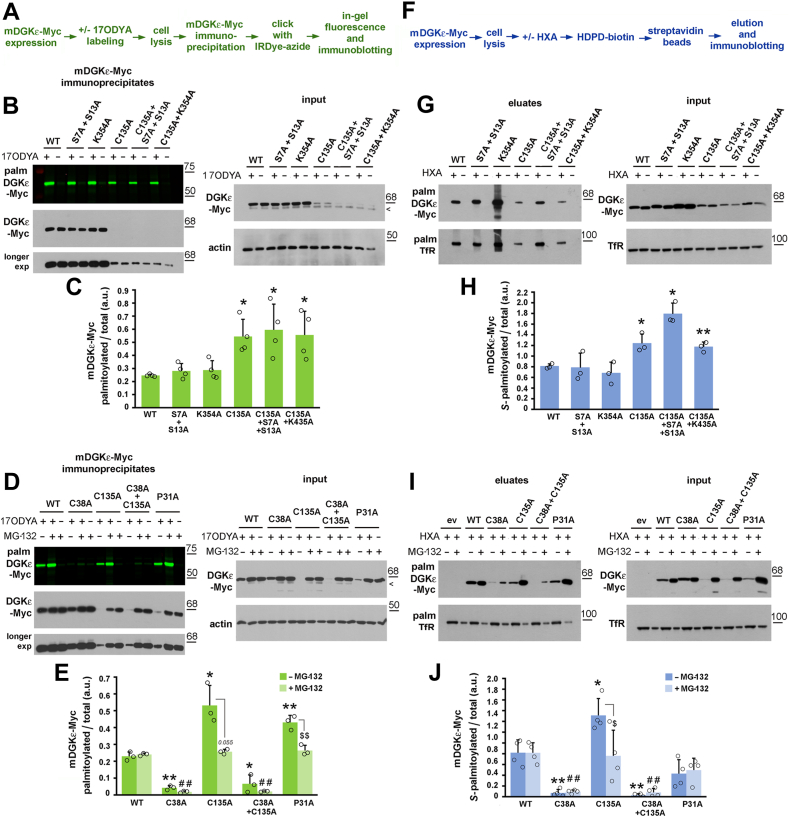
Fig. 2.
Cys38 is the predominant site of mDGKε S-palmitoylation. HEK293 cells were transfected with plasmid encoding WT mDGKε-Myc or its indicated mutant forms and subjected to click chemistry or ABE. A: Scheme of the click chemistry procedure. B–E: After 48 h, cells were subjected to metabolic labeling with 50 μM 17ODYA or exposed to 0.05% DMSO carrier as control (−17ODYA) for 4 h and lysed. D, E: When indicated, prior to lysis, cells were treated with the proteasomal inhibitor MG-132 (1 μM, 18 h). mDGKε-Myc was immunoprecipitated with anti-Myc alpaca antibody and subjected to click chemistry reaction with IRDye 800CW-azide. B, D, upper panels: In-gel fluorescence showing mDGKε-Myc labeling with 17ODYA followed by IRDye-azide. B, D, lower panels: Efficiency of immunoprecipitation determined by immunoblotting with mouse anti-Myc antibody. The content of mDGKε-Myc and actin in input lysates is shown on the right. Arrowheads indicate a band recognized unspecifically by the anti-Myc antibody. C, E: The extent of mDGKε-Myc palmitoylation. mDGKε-Myc fluorescence was determined by densitometry and normalized against the content of respective Myc-tagged DGKε variants in immunoprecipitates. F: Scheme of the ABE procedure. G–J: After 48 h of transfection, cells were lysed and proteins subjected to the ABE procedure involving treatment with HXA (HXA+) or not (HXA−), biotinylation, and capture of originally S-palmitoylated proteins on streptavidin-agarose beads. I, J: When indicated, prior to lysis, cells were treated with MG-132 (1 μM, 18 h). G, I, upper panels: S-palmitoylated mDGKε-Myc eluted from streptavidin-agarose beads revealed with mouse anti-Myc antibody. G, I, lower panels: Transferrin receptor (Tfr), an S-acylated protein, eluted from the beads. The content of total mDGKε-Myc and TfR in input lysates is shown on the right. I: MG-132-treated samples were loaded at half the amount of protein from nontreated samples to avoid overloading. H, J: The extent of mDGKε-Myc S-palmitoylation. The content of mDGKε-Myc in eluates and input lysates was determined by densitometry, normalized against TfR, and the ratio of S-palmitoylated mDGKε-Myc (eluates) to total mDGKε-Myc (lysates) is shown. Molecular weight markers in kDa are shown on the right. Data shown are mean ± SD from four (C, J) or three (E, H) experiments. ∗ and $; ∗∗, ##, and $$, significantly different at P < 0.05 and P < 0.01, respectively, from cells expressing WT mDGKε-Myc (∗, ∗∗), WT mDGKε-Myc in MG-132-treated cells (##). ($, $$) indicate the significance of difference between MG-132-treated and untreated cells. ev, empty vector.