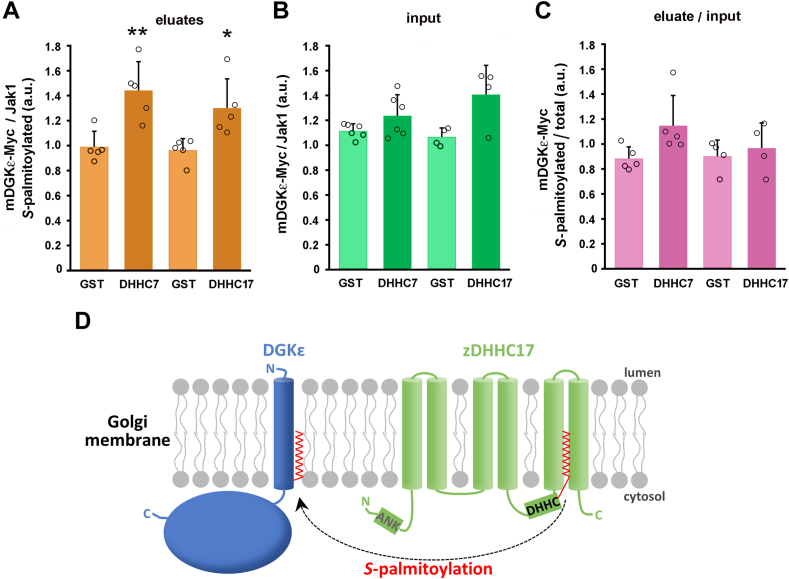
Fig. 4.
DGKε is S-palmitoylated by zDHHC7 and zDHHC17 with a concomitant increase of its abundance. HEK293 cells were cotransfected with plasmids encoding WT mDGKε-Myc and zDHHC7 or zDHHC17 and analyzed as described in Fig. 3. A: The content of S-palmitoylated mDGKε-Myc in eluates was determined by densitometry, as in Fig. 3, and normalized against S-palmitoylated Jak1. B: The content of total mDGKε-Myc in input lysates normalized against total Jak1, as in Fig. 3. C: The extent of mDGKε-Myc S-palmitoylation in cells coexpressing mDGKε-Myc and zDHHC7 or zDHHC17. The content of mDGKε-Myc in eluates and input lysates was normalized against Jak1, and the ratio of S-palmitoylated mDGKε-Myc (eluates) to total mDGKε-Myc (lysates) is shown. Data shown are mean ± SD from at least four experiments. ∗ and ∗∗, significantly different at P < 0.05 and P < 0.01, respectively, from cells coexpressing mDGK-Myc and GST-HA. D: Scheme of DGKε S-palmitoylation by zDHHC17 in the Golgi membrane. S-palmitoylation of DGKε by zDHHC7 likely proceeds analogously. All S-acyltransferases first bind a palmitic acid residue (red) in the DHHC domain and then transfer it to a cysteine residue in the substrate protein. zDHHC17 is unique in that it has six transmembrane helices, whereas most zDHHCs have four. It also has an ankyrin-repeat domain (ANK) at the N terminus involved in interactions with other proteins.