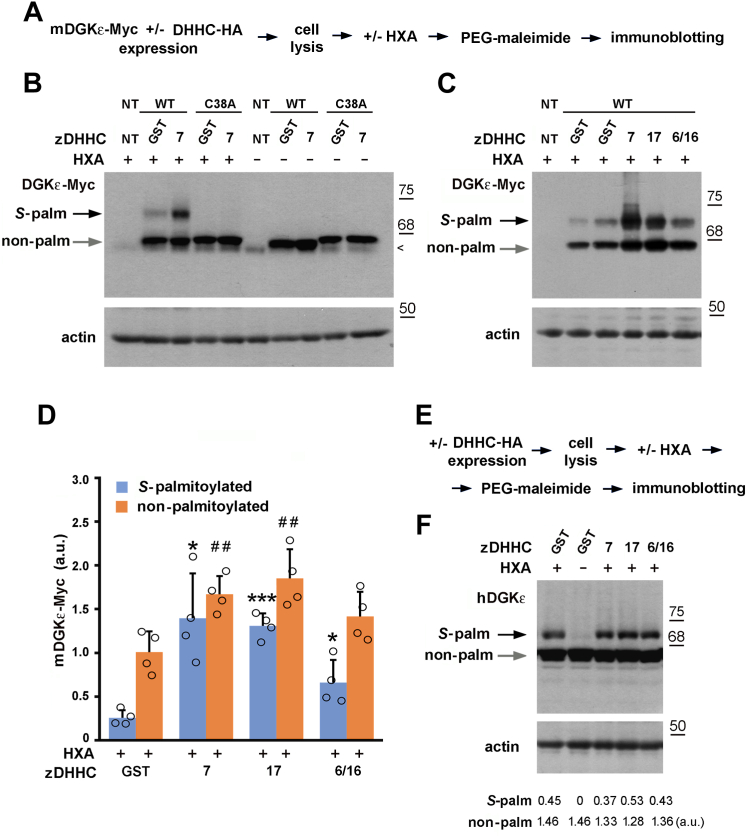
Fig. 5.
zDHHC7, 17, and 6/16 S-palmitoylate DGKε at one cysteine residue. A–D: HEK293 cells were transfected with plasmids encoding WT mDGKε-Myc or Cys38Ala mDGKε-Myc together with indicated zDHHC-HA or GST-HA in controls. After 24 h, cells were lysed and proteins were subjected to the APE procedure involving treatment with HXA (HXA+) or not (HXA−) and incubation with PEG-maleimide. A: Scheme of the APE procedure. B, C: mDGKε-Myc (upper panels) and actin (lower panels) in cell lysates revealed with mouse anti-Myc and mouse anti-actin antibody, respectively. Arrowhead indicates a band recognized unspecifically by the anti-Myc antibody. D: The extent of DGKε S-palmitoylation. The content of PEGylated (originally S-palmitoylated) and nonpalmitoylated mDGKε-Myc was determined by densitometry and normalized against actin. Data are mean ± SD from four experiments. ∗, ∗∗∗, significantly different at P < 0.05 and P < 0.001, respectively, from S-palmitoylated form in cells coexpressing mDGKε-Myc and GST-HA; ##, significantly different at P < 0.01 from nonpalmitoylated form in cells coexpressing mDGKε-Myc and GST-HA. E, F: HEK293 cells were transfected with plasmid encoding indicated zDHHC-HA or GST-HA in controls. After 24 h, cells were lysed and proteins were subjected to the APE procedure. E: Scheme of the APE procedure. F: Endogenous hDGKε (upper panel) and actin (lower panel) in cell lysates revealed with sheep anti-DGKε and mouse anti-actin antibody, respectively. Numbers below blots indicate amount of palmitoylated and nonpalmitoylated endogenous hDGKε determined as in (D). Molecular weight markers in kDa are shown on the right. NT, cells not transfected.