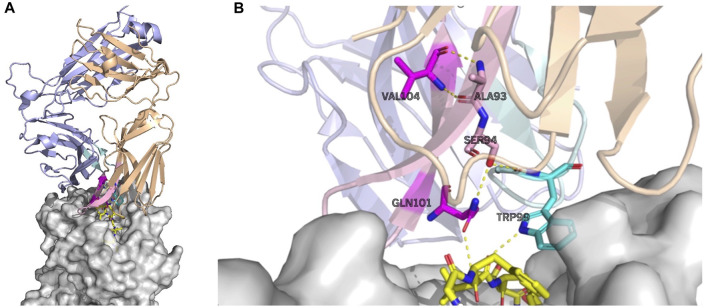
FIGURE 5.
Largely attended residues in the TCR—the influenza virus epitope–HLA complex (PDB ID: 5TEZ)—where the CDR3 sequences of TCR α and β are CAASFIIQGAQKLVF and CASSLLGGWSEAFF, respectively. The left figure (A) shows the overall structure of the complex, and the right figure (B) shows the residue interactions of the largely attended residues: VAL104 (the 14th Val(V) of TCR α) and GLN101 (the 11th Gln(Q) of TCR α) of the TCR α chain, and TRP99 (the 9th Trp(W)) of the TCR β chain. The TCR α chain is wheat, the β-chain is light blue, the TCR α CDR3 part is light pink, and the β CDR3 is pale cyan. The residues with the large attention in CDR3 α are denoted in magenta and that in TCR β CDR3 is cyan. The MHC is denoted in gray. The residues with large attention and interacting residues are represented by sticks. The yellow dot lines represent the hydrogen bonds. VAL104 makes the two hydrogen bonds bind to TCR α ALA93 (the 3rd alanine Ala(A) of TCR α) and may contribute to the stabilization of the end of the CDR3 loop conformation. GLN101 is hydrogen-bonded with TCR α SER94, and SER94 is hydrogen-bonded to TCR β, maintaining the α and β structures. GLN101 of TCR α and TRP99 of β have hydrogen bonds with the epitope. PyMOL (Schrödinger and Delano, 2020) is used for visualization.