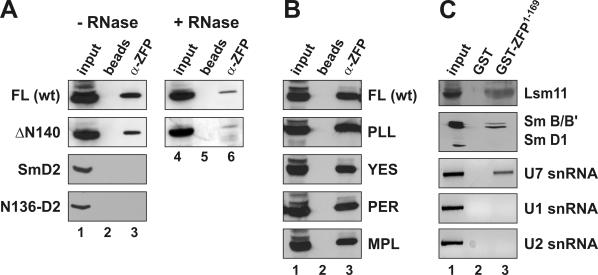
Figure 4.
Determinants of ZFP100–U7 snRNP interactions in mammalian cell extracts. (A) The various HA-tagged proteins indicated on the left were expressed in human 293-T cells by transient transfection. Whole cell extracts were subjected to immunoprecipitation with affinity-purified antibodies directed against the N-terminal 169 amino acids of human ZFP100 (α-ZFP). The samples were subjected to SDS–PAGE, blotted, and the HA-tagged proteins were revealed by anti-HA antibody. For the two top panels, the extracts were incubated in the absence (lanes 1–3) or presence of RNase A (lanes 4–6) prior to immunoprecipitation (see Materials and Methods). The samples for each protein were processed in the same experiment and analysed on the same gel, but an intermediate lane was excised from the picture. FL, full-length murine Lsm11 (HA-mLsm11FL); ΔN140, mLsm11 lacking the first 140 amino acids (HA-mLsm11Δ140); N136-D2, first 136 amino acids of mLsm11 fused to Sm D2 (16). Beads, precipitation by protein G sepharose beads without antibody; input, 1/20 of original extract. (B) Wild-type HA-tagged Lsm11 and the clustered point mutants indicated on the right (for their sequence see Figure 3) were expressed in human 293-T cells and their ability to interact with ZFP100 was assessed by immunoprecipitation with α-ZFP as in (A). (C) Nuclear extract from HeLa cells was subjected to precipitation with glutathione beads coupled to GST-ZFP1–169 (lane 3) or GST alone (lane 2) and analysed for several snRNP components as indicated on the right. Lsm11 was detected by affinity-purified anti-Lsm11 antibodies (16); Sm B/B′ and D1 by the monoclonal anti-Sm antibody Y12; and snRNAs U1, U2 and U7 by RT–PCR as described in Materials and Methods. Input, 1/10 of original extract.