Fig. 7. Fluoxetine diminishes HDM-induced lung inflammation and airway hyperresponsiveness.
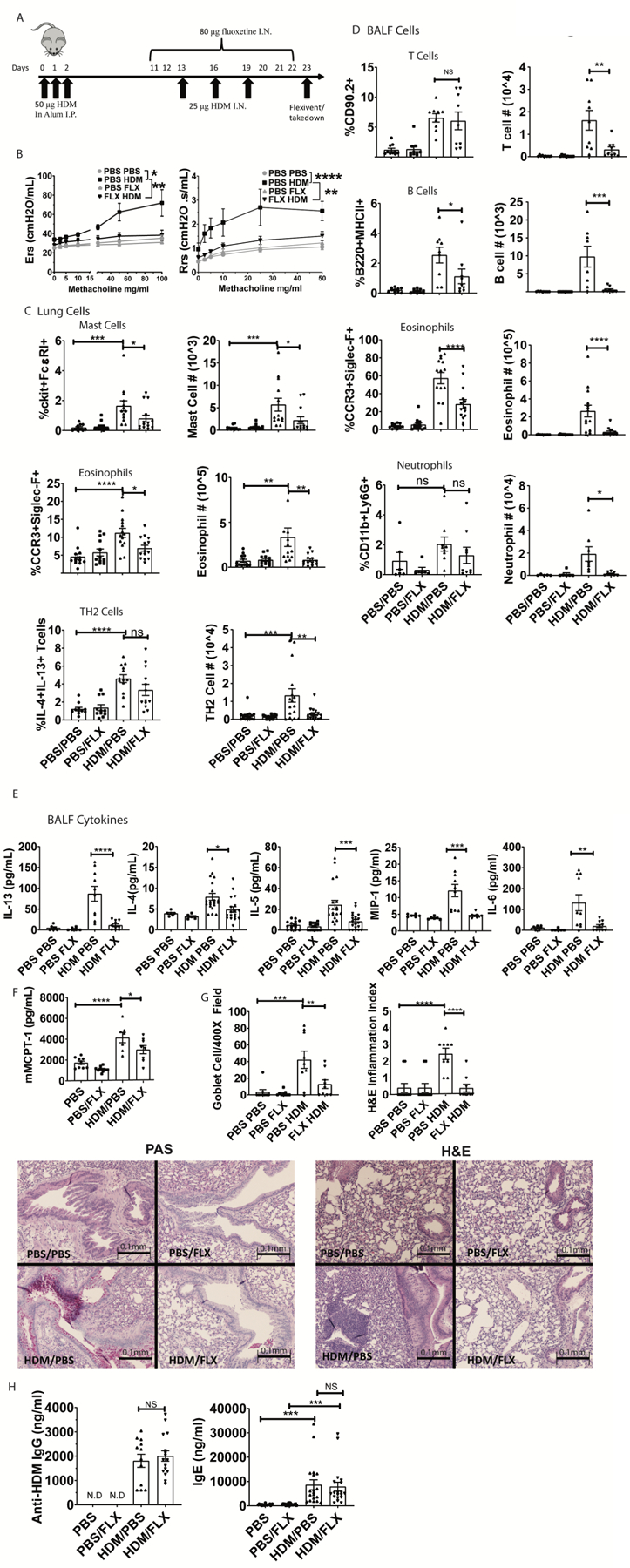
(A) Mice were sensitized and challenged with HDM while receiving fluoxetine or PBS treatments. Animals and tissue were analyzed on day 23. (B) Methacholine-induced lung resistance was measured in anesthetized mice using a Scrieq flexivent instrument. Data are pooled from 2 independent experiments with N=8/group total. (C) Digested lung tissue single cell suspension was analyzed by flow cytometry for mast cells (FcεRI+cKit+), eosinophils (siglecF+CCR3hi) and Th2 cells (CD3+CD4+IL13+IL4+). N=11–14/group. (D) BALF cells were analyzed for T cells (CD90.2+/MHC II-neg), B cells (B220+MHCII+), eosinophils (SiglecF+CCR3+) and neutrophils (CD11b+Ly6G+). Data are pooled from at least 2 independent repeats with each point representing an individual mouse. N=9/group. (E) BALF cytokines were measured by ELISA. Data are pooled from 3 independent experiments. N=10–18/group. (F) Plasma MCPT-1 levels were measured by ELISA from two independent experiments. N=8/group. (G) Lung sections were stained for mucus production with PAS (left) or H&E (right) and blinded samples were scored as described in Materials and Methods. Data are pooled from 2 independent experiments. N=9/group. (H) Anti-HDM IgG and total IgE in plasma were determined by ELISA from two independent experiments. N=11–15/group. All replicates are biological. Statistical values were determined by 1-way ANOVA (Tukey’s test) *p<.05; **p<.001; ****p<.0001 N.D.=not detected.
