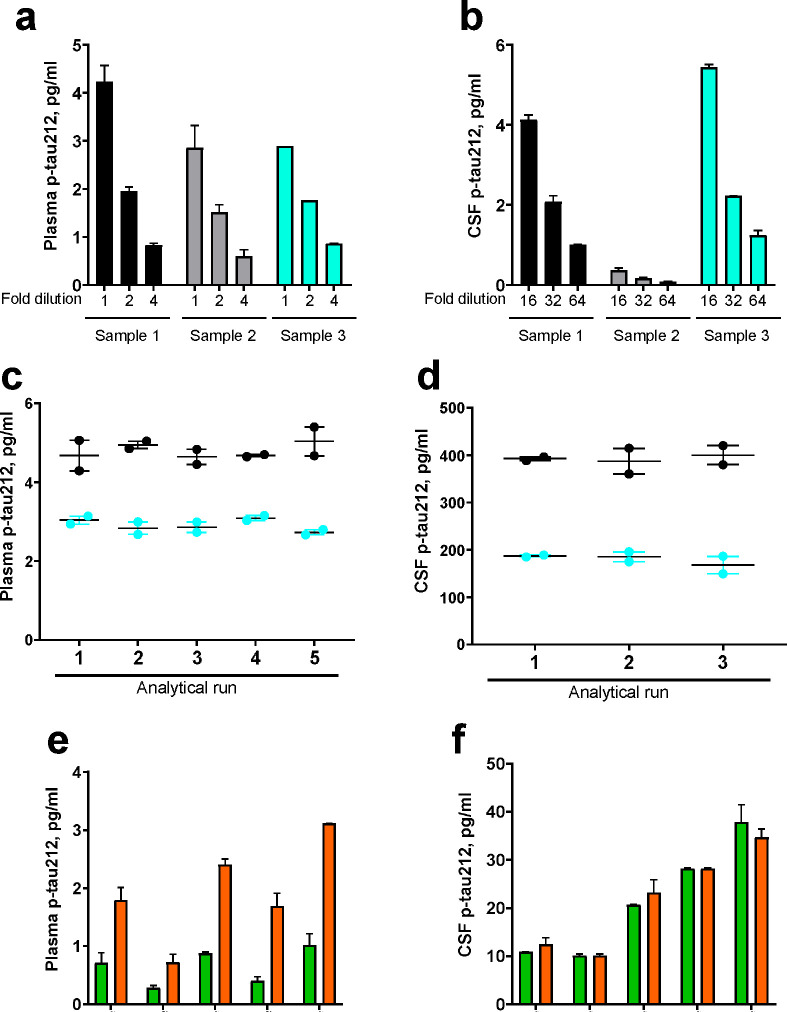
Extended Data Figure 1. Technical validation of the novel plasma p-tau212 and p-tau217 assays developed in this study.
(a) and (b) Dilution linearity of the p-tau212 assay in plasma and CSF respectively. For each matrix, the plots show the measured concentrations in three unique samples with variable levels of the biomarker. Three equal-volume aliquots of each sample were prepared and either measured undiluted or diluted two- or four-fold (16-, 32- and 64-fold for CSF) with the assay diluent. Samples were run in duplicates, aside from plasma sample 3 which was in singlicates. The measured concentrations (without compensation for the fold dilution) are shown. %parallelism values for each subsequent dilution factor are presented in Supplementary Data Table 3. (c) and (d) Within- and between-run stability for plasma p-tau212. The plot shows the concentrations of two separate plasma samples were measured in duplicates in five independent analyses. For the sample with a mean concentration of 2.9 pg/ml, the within-run repeatability was 94.7% and the between-run repeatability was 93.6%. For the sample with mean concentration 4.8 pg/ml within-run and between run stability were both 92.4%. (d) Within- and between-run repeatability of the p-tau212 assay in CSF. The figure shows concentrations of aliquots of two independent CSF samples measured in duplicates in five separate analyses. For the sample with mean concentration 180.1 pg/ml within-run and between run stability were both 90.3%. For the sample with mean concentration 393.3 pg/ml within-run and between run stability were both 93.0%. Note that the concentration values shown in (c) and (d) have been adjusted for the dilution before sample measurement. (e) and (f) Recovery of the p-tau212 assay in plasma and CSF respectively. For each matrix, the plot shows measured concentrations of the samples, concentrations of spiked CSF for plasma recovery or assay calibrator for CSF, and concentrations of samples with added low or high spikes.