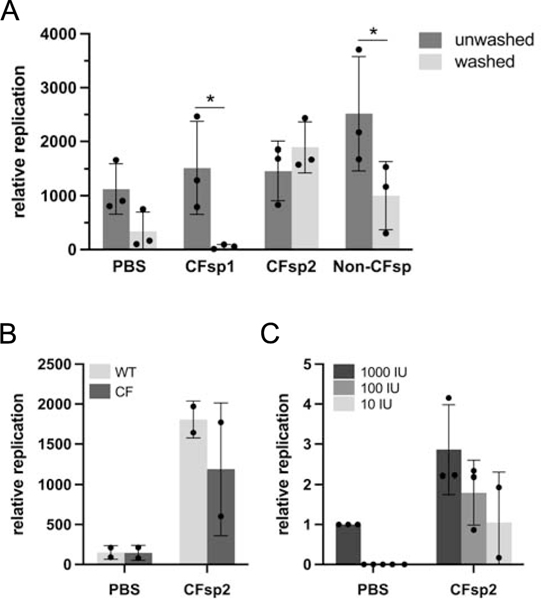
Figure 4.
Effect of CF or non-CF human sputum on SARS-CoV-2 infection. Primary bronchial epithelial cells were prepared as epithelial monolayers at air-liquid interface. (A) A subset of bronchial epithelial cell filters were washed to remove conditioned surface liquid and mucus. Prior to infection, 2,000 infectious units of SARS-CoV-2 were diluted 1:5 either in PBS or in freshly expectorated mucus collected from patients with cystic fibrosis or a healthy individual and added to cells in culture. Multiple comparison p values washed versus unwashed (Bonferroni): PBS: 0.57; CFsp1: 0.05; CFsp2: >0.99; Non-CFsp: 0.04. (B) Paired hBEC samples (WT/WT or F508del/F508del) were washed to remove surface liquid and mucus. SARS-CoV-2 viral stock was diluted 1:5 in PBS or sputum supernatant and 2,000 infectious units added to airway cell monolayers. PBS versus CFsp2, p = 0.01 (2-way ANOVA). (C) Prior to infection of unwashed non-CF epithelia, 1,000, 100 or 10 infectious units (IU) of SARS-CoV-2 were diluted in PBS or CF sputum supernatant 2. PBS versus CFsp2, p = 0.02; for virus dose effect, p = 0.03 (2-way ANOVA). Replication values in all panels represent viral RNA levels at 48 h. In (C), RNA levels are additionally normalized to the sample inoculated with 1,000 IU virus in PBS. Error bars in all panels represent standard deviations.