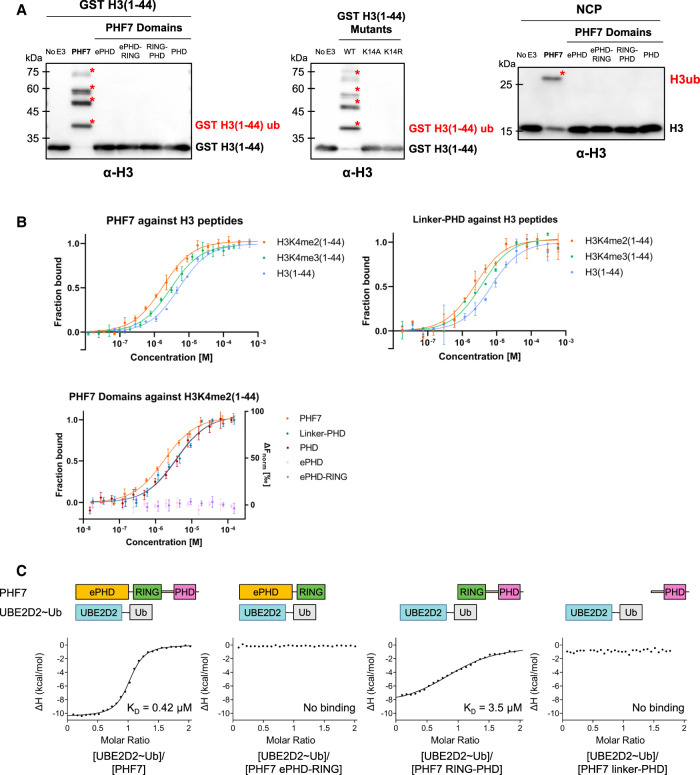
Figure 2.
PHF7 uses the PHD for histone H3 substrate binding and RING–linker–PHD for E2 interaction. (A) In vitro ubiquitination assay with the indicated PHF7 domains using GST-tagged H3(1–44), H3(1–44) mutants, or the NCP as the substrate. Red asterisks mark ubiquitinated H3. The antibody used is specific against histone H3. (B) MST binding curves showing the fraction of bound H3(1–44) peptides (unmodified, K4me2, and K4me3) against different PHF7 constructs. For the ePHD and ePHD–RING, a baseline level of ΔFnorm (right axis) indicates that binding cannot be detected. Error bars represent the standard deviation of three technical replicates. (C) Isothermal titration calorimetry binding curves of the indicated PHF7 domains with UBE2D2∼Ub. The resulting KD values are indicated.