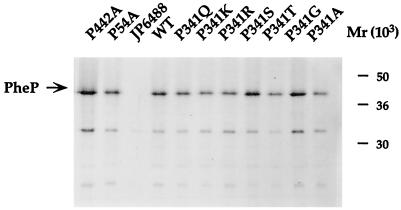
FIG. 2.
Comparison of levels of wild-type (WT) and proline mutant PheP proteins produced by [35S]methionine-cysteine-labelled JP6488 cells by immunoprecipitation. Representative mutant proteins were pulse-labelled for 1 min before being solubilized with NaOH and immunoprecipitated with an antibody prepared against a synthetic PheP peptide. The radioactivity in gels of immunoprecipitated PheP proteins was quantitated as described in Materials and Methods. Similar values were obtained in two separate experiments.