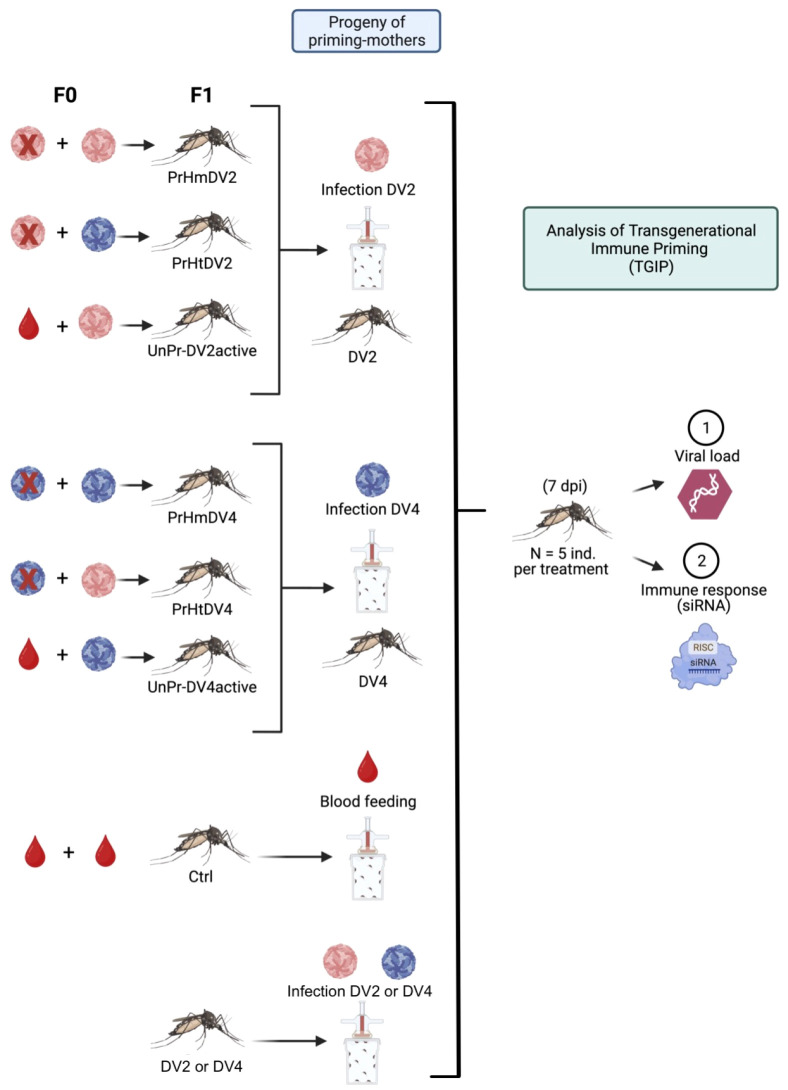
Figure 3.
Experimental design of the transgenerational immune priming (TGIP) analysis of the progeny (F1) of priming mothers (F0) with homologous (PrHm) and heterologous (PrHt) infections with dengue virus serotypes 2 (DV2, represented in red) and 4 (represented in blue) and unprimed (UnPr) mosquitoes but infected with a second challenge with active dengue virus (UnPr-DV2active or UnPr-DV4active respectively). As DV infection control groups, progeny (of mothers without infection with DV) were infected with DV2 or DV4, respectively. In addition, another control group fed with rabbit blood without infection (Ctrl) was added. At seven days post-infection (dpi), five individuals per treatment were collected to evaluate the viral load and antiviral immune response (siRNA).