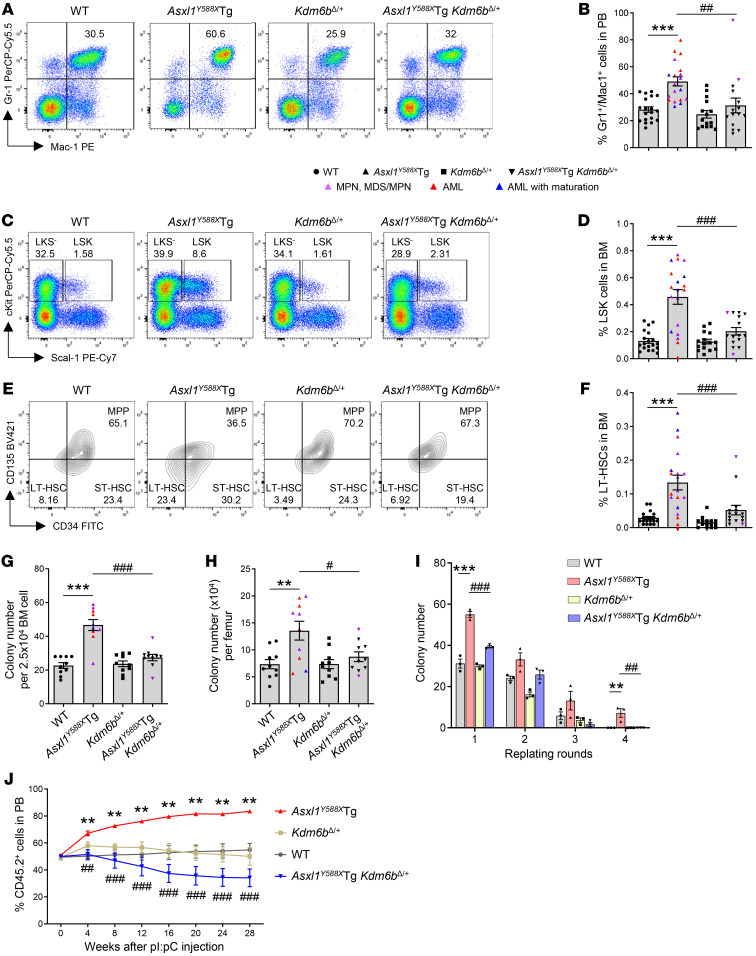
Figure 3. Heterozygous deletion of Kdm6b restores ASXL1aa1–587–mediated HSC phenotypes and myeloid differentiation.
(A) Flow cytometric analysis of myeloid cells in PB from representative mice of each genotype. (B) The frequencies of Gr1+Mac1+ cells in PB from WT (n = 19), Asxl1Y588XTg (n = 19), Kdm6bΔ/+ (n = 15), and Asxl1Y588XTg Kdm6bΔ/+ (n = 15) mice. (C and E) Flow cytometric analysis of HSPCs in BM cells from representative mice of each genotype. (D and F) Quantification of the percentages of LSK cells (D) and LT-HSCs (F) in BM from WT (n = 19), Asxl1Y588XTg (n = 19), Kdm6bΔ/+ (n = 15), and Asxl1Y588XTg Kdm6bΔ/+ (n = 15) mice. (G and H) Colony-forming assay using BM cells from WT, Asxl1Y588XTg, Kdm6bΔ/+, and Asxl1Y588XTg Kdm6bΔ/+ mice (n = 10 per genotype). (I) Serial cell replating assays using whole BM cells (n = 3 mice per genotype) were performed to determine HSC self-renewal capability. The cells were replated weekly for 4 weeks. (J) Percentages of donor-derived CD45.2+ cells in the PB of recipient animals at indicated time points (n = 5 per genotype). Data represent the mean ± SEM. **P < 0.01 and ***P < 0.001 vs. WT mice, and #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. Asxl1Y588XTg mice, by 1-way ANOVA with Tukey’s multiple-comparison test (B, D, and F–J).